J.ophthalmol.(Ukraine).2021;2:46-54.
|
http://doi.org/10.31288/oftalmolzh202124654 Received: 10 December 2020; Published on-line: 19 April 2021 Angioid streaks of the retina A. R. Korol, V. V. Rostel SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE: Korol AR, Rostel VV. Angioid streaks of the retina. J.ophthalmol.(Ukraine).2021;2:46-54.http://doi.org/10.31288/oftalmolzh202124654 This paper is a review of the current literature on the etiology, pathogenesis, clinical picture and treatment of angioid streaks of the retina. Angioid streaks (AS) are a disease of the fundus characterized by cracks in the thickened, calcified and abnormally brittle collagenous and elastic layers of the Bruch's membrane; in up to 50% of cases, associated with a systemic condition, most commonly pseudoxanthoma elasticum (PXE), Paget's disease of bone, and etc. Various diagnostic techniques (visual acuity testing, refractometry, tonometry, slit-lamp examination of the anterior segment, ophthalmoscopy, retinal fluorescein angiography (FA) and optical coherence tomography (OCT)) are used for the diagnosis, assessment, and monitoring of the disease. Choroidal neovasculatization (CNV) is the most common complication leading to reduced vision. Intravitreal anti- vascular endothelial growth factor administration is currently the most effective treatment for CNV. Keywords: angioid streaks, choroidal neovascularization, intravitreal administration
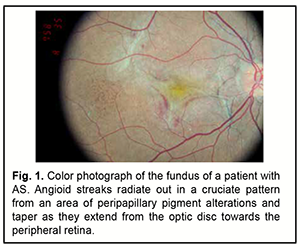
Angioid streaks (AS) are a disease of the fundus characterized by cracks in the thickened, calcified and abnormally brittle collagenous and elastic layers of the Bruch's membrane. They were first described by Doyne in 1989 as irregular radiating lines extending from the optic disc to the peripheral retina found in eyes with peripheral hemorrhages after blunt trauma [1]. In 1892, Knapp was the first to coin the term “angioid streaks” because they can closely resemble blood vessels [2]. Not until 1917 did Kofler correctly determine that angioid streaks represented changes at the level of Bruch's membrane [3]. Clinical examination with subsequent histopathological findings by Bock [4] in 1938 in two patients with pseudoxanthoma elasticum confirmed that the underlying abnormality was not vascular in nature but rather a structural alteration in Bruch’s membrane. A few years later similar histopathological results were found in patients suffering pseudoxanthoma elasticum, Paget’s disease, but also from systemic diseases [5]. Etiology and epidemiology As much as 50% of all cases occur idiopathically [6]. Angioid streaks may be found most often in pseudoxanthoma elasticum (PXE or Grönblad–Strandberg syndrome) which is characterized by progressive calcification and degeneration of elastic fibers. The name comes from Ester Elisabeth Grönblad (1898-1942), a Swedish ophthalmologist, and James V Victor Strandberg (1883-1942), a Swedish dermatologist who discussed the association between eye and skin findings in PXE [7]. The frequency of AS in patients with PXE varies between 59% and 87%, based on whether the pseudoxanthoma was diagnosed clinically or with skin biopsy. Practically all PXE patients will have developed angioid streaks 20 years after first diagnosis [8]. Other associations of angioid streaks include [8, 9]: ● Ehlers Danlos syndrome ● Marfan syndrome ● Paget's disease of bone- Angioid streaks are noted in up to 10% of cases. ● Sickle cell disease- Angioid streak is noted in around 1-2% cases, usually after the 3rd decade. Angioid streaks have been reported in sickle cell trait, homozygous sickle cell disease, sickle cell-thalassemia, and sickle cell hemoglobin C disease. ● Other hemoglobinopathies- acquired hemolytic anemia, hereditary spherocytosis, beta thalassemia major, beta thalassemia minor, beta thalassemia intermedia, hemoglobin H disease, and congenital dyserythropoietic anemia type 1. ● Acromegaly, ● Hemochromatosis ● Diabetes mellitus, ● Sturge Weber syndrome with facial angiomatosis, ● Myopia ● Hyperphosphatemia ● Neurofibromatosis ● Hypercalcemia ● Abetalipoproteinemia ● Familial polyposis of the colon ● Congenital hypertrophy of retinal pigment epithelium ● Diffuse lipomatosis ● Cutaneous calcinosis ● Microsomia ●Trauma ● Hypertensive coronary disease ●Senile elastosis ●Epilepsy Some authors, however, noted that angioid streaks are not a common feature of Ehlers Danlos syndrome, and have questioned the association of the two conditions [10, 11]. The exact prevalence of PXE is unknown, but estimates of 1:20,000 to 1: 100,000 live births have been quoted. That is, estimates suggesting that the global prevalence is 1 in 50,000 would imply that there could be as many as 150,000 patients affected with PXE worldwide. Even this number may be an underestimate, since PXE, like many genetic conditions, has variable expressivity and its milder forms often remain undiagnosed for years or even for the lifetime [6, 12]. PXE has been suggested to be more severe in women, and female/male incidence rate was reported as 2:1[13]. The inheritance of PXE is autosomal recessive and the causative mutation for PXE has been localized to the ABCC6 gene transporter protein on chromosome 16. Mutations in the ABCC6 gene lead to reduction or absence of the transmembrane transport ADP dependent protein (MRP6), causing an accumulation of extracellular material [6, 14]. The ABCC6 gene in patients with AS was scanned for putative mutations by single-strand conformation polymorphism technique, and a specific base substitution of c.G3803A was identified in exon 27, resulting in a change in codon from CGG to CAG (p.R1268Q). The authors concluded that the missense mutation p.R1268Q is associated with the disease state of AS [15]. Subsequent examination of a cohort of 54 Japanese patients with AS, identified six mutations (p.R419Q, p.E422K, c.2542delG, Del_exon23, c.3774-3775insC, and p.E1427K) as causal mutations for this eye disease [16]. Кatagiri and colleagues [17] concluded that the results of their study indicated that ABCC6 variants play a significant role in patients with AS in the Japanese population. They found four variants (p.V848CfsX83, p.Q378X, p.R1357W and p.R419Q) to be significantly associated with AS, and their findings suggested that the ABCC6 variant spectrum differs between Asian and European populations. They also concluded that AS are a hereditary retinal disorder, although the inheritance pattern has not yet been fully elucidated [17]. Pathogenesis and histology The pathogenesis of AS, especially in patients with pseudoxanthoma elasticum (PXE), is based on mineralization of the elastin-rich Bruch's membrane due to the absence of systemic antimineralization factor(s), which leads to calcification of elastic fiber-rich connective tissues, particularly, those of the membrane. The condition can result in rupture of blood vessels, leading to further deterioration of the visual function. Pathogenetically, the development of AS is believed to be as follows. The early stages are characterized by BM thickening and/or reduction in pigment granule size, and redistribution of pigment in the retinal pigment epithelium (RPE). These abnormalities may be accompanied by changes in the above-lying retinal layers and underlying choroidal capillary layer. Subsequently, the disease can progress to the development of subretinal neovascular membrane (SNM) due to fibrovascular tissue growth through the breaks of Bruch's membrane resulting from changes in vascular endothelial growth factor (VEGF) activity. A disciform scar develops in the presence of the SNM in the terminal stage of untreated disease [18]. The following histopathological abnormalities are noted in angioid streaks: thickening and mineralization of the Bruch’s membrane; well-delineated crack-like breaks in the collagenous and elastic layers of Bruch's membrane; neovascular or fibrovascular tissue may develop at the margin of Bruch's membrane and grow beneath the retina or the RPE. Actually, angiois streaks are breaks in the Bruch's membrane [19]. The fragility and opacification of the Bruch's membrane may be primarily due to the degeneration of the elastic portion of Bruch's membrane along with deposition of calcium, magnesium, or iron salts due to disturbed metabolism. These changes appear mostly peripapillary and do not involve the ora serrata. The orientation of angioid streaks may be related to force lines due to the pull of intrinsic and extrinsic muscles of the eye around a fixed location (e.g., optic nerve) [5]. In PXE, the elastic tissue in the aorta shows thickening and disintegration, an there is degeneration of the choriocapillary and ciliary network [20]. Clinical picture Although commonly the diagnosis is not difficult, angioid streaks can sometimes be confused with retinal vessels on non-meticulous and/or hasty ophthalmoscopy. AS are asymptomatic unless the fovea is involved, and found incidentally during a routine eye examination. If the macula (especially, the foveola) is involved, patients with AS may present with decreased vision and metamorphosia. In addition, these patients may present with decreased vision if CNV and/or AS develop at the foveola, leading to subretinal hemorrhages. These hemorrhages are commonly located in the posterior pole and resolve spontaneously [21, 22]. Patients with AS associated with CNV complain of decreased vision and metamorphosia. Subretinal neovascular membranes are commonly located adjacent to AS. CNV represents a major cause of severe visual loss in patients with angioid streaks. While type 2 СNV is the most common presenting anatomical subtype of NV to occur in the setting of angioid streaks, type 1 and mixed (type 1 and type 2) lesions occur in up to 16% of eyes [23]. The complaints characteristic of AS-associated CNV are made earlier than those of exudative age-related macular degeneration (AMD)-associated CNV [14]. If untreated, the growth of choroidal neovascular membranes results in subretinal scarring, leading to significantly decreased vision. An associated feature of AS is a 'peau d'orange' fundus appearance: there is pigmentary mottling (small areas of pigmentation which are sometimes confluent at the level of retinal pigment epithelium interspersed with normal fundus color) specifically temporal to the fovea sometimes reaching the equator, giving an appearance of the skin of an orange. Changes that extend to the optic disc nasally are rarely seen, and are less common in AS associated with sickle cell anemia or Paget’s disease. Peau d'orange appearance of the fundus is noted in children even before the development of angioid streaks. AS appear as narrow, jagged lines deep to the retina, almost always bilaterally. Angioid streaks typically radiate out in a cruciate pattern from an area of peripapillary pigment alterations (and taper as they extend from the optic disc towards the peripheral retina), although they may form a circumferential ring around the peripapillary area as well (Fig. 1).
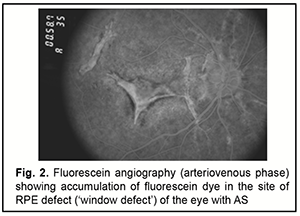
AS are evident in fundoscopy a few millimetres from the optic disc and rarely occur in the periphery of the posterior pole. They are clinically characterized by a variable caliber (50-500 μm), serrated margins of the streaks, and subretinal location (the retinal vessels are superficial to the angioid streaks). The color of AS depends on the background coloration of the fundus and the degree of atrophy of overlaying RPE. Thus, angioid streaks are red in light-colored individuals, while in patients who have darker background pigmentation, they are usually medium to dark brown. With age, they become darker, whereas the RPE becomes less colored. In addition, with age, the number, width, and length of angioid streaks may increase. Sometimes AS are extremely dark and have several bonds between them giving the appearance of a 'spider's web' in the retina. In other occasions a fibrous connective tissue develops around angioid streaks which appear obscure and light-colored [7, 24, 25]. Other typical ocular manifestations of angioid streaks include: ● Optic nerve head drusen or hyaline bodies are seen in about 5% cases with PXE and angioid streaks, the disc is hyperautofluorescent and shows hyperechoic lesion with acoustic shadow on ultrasound of the optic nerve head. ● Macular thinning or pigmentary changes- The changes are usually bilateral, and there is no hemorrhage or hard exudates. ● Pattern dystrophy of the macula (including fundus pulverulentus, butterfly-shaped dystrophy, fundus flavimaculatus, reticular dystrophy, and vitelliform pattern dystrophy). The type of pattern dystrophy may change with time, and the changes of pattern dystrophy may appear on follow-up. ● Small round RPE atrophic patches- These may have a white, yellow, or pink color. Punched out scars similar to presumed ocular histoplasmosis syndrome (POHS) may also be seen. ● Crystalline bodies or whitish subretinal round lesions in mid-peripheral fundus usually inferiorly- Some of these nodules have Comet-like tail of depigmentation. Crystalline body is seen in up to 75% of cases. ● Atrophy of RPE near the angioid streak [26] ● Retinal telangiectasis can be associated with AS in elderly patients and in sickle cell haemoglobinopathy [27]. ● Polypoidal choroidal vasculopathy [28, 29] ● Traumatic rupture of the Bruch’s membrane or choroid [22]. The following symptoms are seen in PXE-associated AS: ● Skin lesions (discrete in early-stage and later confluent yellowish papules or pseudoxanthomas) at the sides of the neck, umbilical region, and flexures like elbow giving a 'plucked chicken' appearance ● Gastrointestinal bleeding ● Intermittent claudication/ peripheral arterial vascular disease ● Coronary artery disease ● Aneurysms at brain, kidney, mesentery causing hemorrhage ● Brain infarct or hemorrhage [7]. Between 72% and 86% of patients with AS will develop CNV in one eye, with 71% going on to develop CNV in the other eye, after roughly 18 months [2, 30]. The risk of developing CNV increases with age. Other risk factors comprise the width, length and location of the AS. The wider and longer are the angioid streaks the higher the risk for CNV and especially if the lesions are located in a distance less than one optic disc diameter from the foveola [31]. Angioid streaks associated with PXE have a relatively high probability of developing macular CNV; the opposite happens in patients suffering sickle-cell anemia [31, 32]. Macular CNV is the major cause of severe visual loss in these patients [33]. The major problem in managing CNV in eyes with AS is recurrence. In several years, subretinal neovascularization may recur or new CNV may develop at distant sites. Final visual acuity depends mainly on the patient’s age at the onset of symptoms as well as the presence of any systemic disease [22]. As discussed above, the disease has a progressive course; the following stages are distinguished: Stage 1 (asymptomatic): AS are asymptomatic and are found during a routine fundus examination, eye functions are preserved, and patients may have complaints related to the foveal Bruch’s membrane defect; Stage 2: patients have decreased vision and metamorphosia related to subretinal neovascular membranes; angioid streaks present with decreased vision and metamorphosia related to subretinal neovascular membranes; Stage 3: patients have significantly decreased vision (as low as a few hundredths of a unit), with their eyes showing extensive choroidal atrophy [34]. Diagnosis Patients with AS should receive a comprehensive eye examination including visual acuity testing, refractometry, tonometry, slit-lamp examination of the anterior segment, ophthalmoscopy, retinal fluorescein angiography (FA) and optical coherence tomography (OCT). Although angioid streaks are usually diagnosed based on ophthalmoscopy (Fig. 1), FA may be helpful in the diagnosis if ophthalmoscopic findings are inadequate or doubtful (Fig. 2).
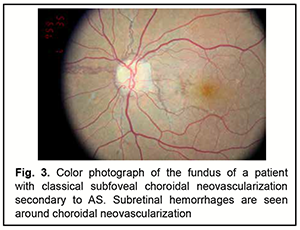
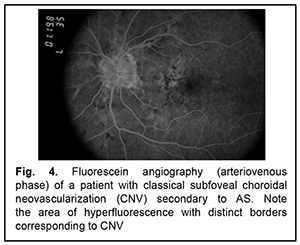
Early fluorescence and accumulation of fluorescein dye in the site of RPE defect (‘window defect’) are usually seen on FA of eyes with AS. Fluorescein leakage is evident when CNV (Figs 3, 4) is present [35].
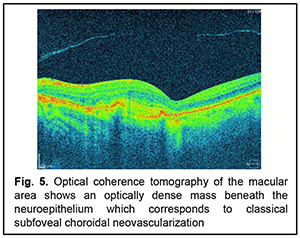
On fundus autofluorescence imaging, AS appear as hypoautofluorescent fissures, sometimes showing expansion of the hypoautofluorescence suggestive of RPE absence or atrophy. Drusen of the optic nerve are frequently associated with AS and appear as autofluorescent bodies [36]. Abnormalities of the RPE-photoreceptor complex detected by FAF imaging were more diverse and widespread than those found by fundoscopy or FA [37]. The angioid streaks having adjacent RPE alterations appear brick red, and infrared imaging showed them as well-demarcated dark fissures, even when these passed unnoticed on funduscopy [38]. Indocyanine green angiography (ICGA) can be helpful in some rare cases when ophthalmoscopy and FA cannot confirm the diagnosis of AS. Such occasions involve the severe lesions of RPE which cause hyperfluorescent lines that are obscure or the development of macular CNV; in these cases ICGA demonstrates the neovascular membrane more clearly than FA. ICGA shows hyperfluorescent lines with ‘pinpoints’ over their whole length that are larger and more numerous than those on fluorescein angiography or red-free photography. On the contrary, recently developed angioid streaks become evident only at the late stage of the examination and have the appearance of hypofluorescent linear distortions around optic nerve head or posterior pole. Although Lafaut and colleagues [39] concluded that ICGA outlined angioid streaks better than FA in the majority of cases, and occult choroidal neovascularization was better defined by ICGA, in their study, 18% of funduscopically visible streaks could not be visualized by ICGA. OCT is a current non-invasive method for diagnoisis of various fundus disorders [40, 41]. In a study by Marchese and colleagues [42], Bruch’s membrane undulations, mostly observed around the optic nerve head, were found in 19 (59.4%) of 32 eyes with AS. In addition, evolution of BM undulations into Bruch’s membrane breaks was observed in 5 eyes (15.6%). Moreover, CNV was observed in 12 eyes (37.5%) during follow-up, typically in areas of Bruch’s membrane interruption (Fig. 5).
In a study by Ellabban and colleagues [25], the macular area of 39 eyes of 23 patients with AS and of 20 normal eyes of 20 matched controls (Group 1) was studied with a swept source OCT system. Eyes with angioid streaks were classified into 1 of 4 groups: those without choroidal neovascularization (CNV) (Group 2); those with CNV that had no history of treatment (Group 3); those with CNV that had previously received only anti-vascular endothelial growth factor treatments (Group 4); and those with CNV that had previously received photodynamic therapy (Group 5). Mean choroidal thickness of the macula in Group 2 (218.9 ± 46.8 μm) was as great as that in Group 1 (218.8 ± 69.2 μm). However, the macular choroidal thickness in Group 3 (119.7 ± 49.2 μm), Group 4 (140.1 ± 64.9 μm), and Group 5 (144.0 ± 52.6 μm) was significantly less than that of Group 1 (P < .05). There were no statistical differences between Groups 3 through 5. In each group, the choroid of the nasal quadrant was significantly thinner compared to that in other quadrants (P < .05). Multimodal imaging in combination with optical coherence tomography angiography (OCTA) provides us with an excellent tool for the early detection and monitoring of AS related CNV [43, 44]. In a study by Chapron and colleagues [43], OCT-A was able to detect CNV associated with AS in 87.5 % (28/32) eyes. OCT-A phenotypes of CNV were classified into interlacing pattern in 9 eyes, pruned vascular tree pattern in 7 eyes, and combined pattern in 12 eyes. Moreover, 6/32 eyes (18.75%) presented a dark area surrounding the neovascular complex (“dark halo”). All eyes that presented a dark halo received an anti-VEGF treatment in the past 6 months (p = 0.024) and 4/6 eyes (66.7%) had exudative signs (p = 0.165). There was a statistically significant association between antiangiogenic treatment and pattern type, as follows: recent anti-VEGF treatment (<6 months) was associated with both the interlacing and combined patterns, while in the eyes without treatment in the last 6 months the pruned vascular tree pattern was prevalent (p < 0.001). Differential diagnoses of angioid streaks include [7]: ● Normal retinal vessels ● Lacquer's cracks- A degenerative finding noted in pathological myopia, also characterized by breaks in the Bruch's membrane. It may be associated with subretinal hemorrhage without evidence of CNV. ● Reticular dystrophy of the retinal pigment epithelium (RPE)- It is characterized by RPE changes that cause pigmentation in 'fishnet with knot' pattern. The changes are located at the posterior pole and may cause a round circle similar to angioid streaks. ● Subretinal tracks due to ophthalmomyiasis interna are smooth and may cross each other multiple times. ● Subretinal bands may be noted in long-standing retinal detachment or operated retinal detachment cases, which may simulate angioid streaks. ● Bilateral disciform scars may be seen in wAMD. ● Peripheral punched out scars may simulate presumed ocular histoplasmosis syndrome. Prognosis Angioid streaks of the fundus are not apparent at birth. Mansour and colleagues [45] examined in a retrospective manner the fundus pictures of 111 subjects with AS. The earliest form of AS became apparent at age 8 with findings of narrow short radial discontinuous hypopigmented streaks. Thereafter AS enlarged in length and width. The end-stage was disciform macular degeneration, helicoid peripapillary atrophy, or diffuse choroidal sclerosis with obscuration of the AS. The visual prognosis of untreated patients with choroidal neovascularization is poor. Systemically, though most patients with pseudoxanthoma elasticum have a normal life span, early death can occur due to gastrointestinal hemorrhage, cerebral hemorrhage, and myocardial infarction [8]. Treatment Angioid streaks are usually asymptomatic and do not need any treatment. However, these eyes are more prone to develop subretinal hemorrhage after trivial trauma and the clinical team, and the patient should be educated to use protective eyewear. All patients with angioid streaks should be screened for potential systemic associations. Examination of family members may give clue to the systemic disease. In cases with subretinal hemorrhage, a fundus fluorescein angiogram should be performed to rule out CNV. If CNV is absent, the hemorrhage usually resolves on its own. If CNV is detected, the management options include the following. Laser photocoagulation Clarkson and colleagues [46] presented a case series report of six patients with angioid streaks and CNV of the macular region, who were treated with a thermal laser directly targeting the neovascular membrane. Their results were devastating and all patients developed further expansion of the neovascularization causing loss of central vision. At the same time, other small case series reports stated that laser photocoagulation had some encouraging results in neovascularization outside the foveolar region [47, 48, 49]. In 1988, Gelisken and colleagues [50] presented the results of a study involving 30 eyes with CNV due to angioid streaks. All patients were treated with green argon or krypton laser and the follow-up was two months to 16 years. The authors concluded that the eyes which had extrafoveolar neovascular membranes benefited from the application of thermal laser since they retained a useful vision compared to the eyes that remained untreated. Additionally, they stressed that in cases of subfoveal CNV, no treatment should be applied. Finally, they suggested that no statistically significant difference was observed in using argon and krypton laser, but they preferred the latter. During the last 20 years, several clinicians drew the same conclusion from these results and thus the dominant contemporary theory is that the efficacy of photocoagulation for macular CNV in angioid streaks is of limited application due to the high percentage of neovascular membrane relapse. Transpupillary thermotherapy (TTT) The diode laser used in TTT beam causes less absorption by the RPE and deeper penetration in the choriocapillaris. There have been reports on the use of TTT for the treatment of AS-associated CNV, but treatment outcomes were poor. Aras and colleagues [51] tried this method in patients with SNM in AS and concluded that it does not seem to affect the course of the disease and at the same time they observed a spreading out of the borders and the leakage of the membrane. Macular translocation surgery This surgical technique was introduced by Machemer and Steinhorst [52] in 1993. Macular translocation involves moving the macula away from the ingrowth of the new vessels. it is a complex, difficult, long-lasting operation which has serious complications (retinal detachment, proliferative vitreoretinopathy, endophthalmitis, etc.) threatening the central and peripheral vision of the patients. Roth and colleagues [53] have reported on the use of macular translocation surgery in macular SNM in angioid streaks. They described a case where they performed a successful lower macular translocation followed by laser photocoagulation in the region of SNM in a patient with AS. The end result was encouraging, the visual acuity of the patient improved from 20/125 to 20/40. Other surgeons had similarly good results [53, 54]. These encouraging results, however, can not be fully evaluated since the number of patients and the studies involved are of a very small number. Photodynamic therapy Large randomized clinical trials were performed to evaluate the efficacy of photodynamic therapy (PDT) with verteporfin for CNV secondary to AMD and pathologic myopia. Since the results of these studies were promising, clinician tried to use PDT in other pathological entities causing CNV. In 2000, the Archives of Ophthalmology published the first results of the application of PDT for CNV not caused by AMD [55]. In this study group there was one patient with AS. He was a 55-year-old man who was treated only once with PDT and had a 12-month follow up. During the follow up time there was no further deterioration of his visual acuity, but at the same time the leakage of fluorescein dye from the neovascular membrane remained unaffected. Subsequent studies reported contradicting results on the use of PDT for CNV [56, 57]. In 2004, Menchini and colleagues [58] published the results of a retrospective multicenter clinical trial from Italy, which involved 40 patients with AS who developed CNV in the macula and were treated with PDT. Visual acuity reduced and CNV had increased in size during the last follow-up visit in almost two thirds of the patients (62%). Others [59] have reported similar treatment outcomes. Ladas and colleagues [60] evaluated the effectiveness of conventional PDT in a series of 24 eyes of 22 patients with CNV due to AS and compared it to the effectiveness of a PDT modification where retreatments were performed earlier (every 8 weeks instead of 12). The authors concluded that the functional and the anatomic results of PDT were not satisfactory, even when retreatments were performed earlier than the conventional time. Therefore, from all previous studies and case reports it is evident that, despite the initial encouraging results from the application of PDT for the treatment of CNV in the macula, the end results did not fulfill the initial expectations. Anti-vascular endothelial growth factor (VEGF) treatment Anti-vascular endothelial growth factor (VEGF) treatment (especially, early on) has resulted in unprecedented visual and anatomic outcomes far outpacing other available treatments for CNV due to AMD or pathological myopia [61, 62, 63]. In 2009, Wecke and colleagues [64] reported favorable result in a patient with CNV due to angioid streaks after intravitreal injection of bevacizumab. Chang and colleagues [65] reported their results of intravitreous injection of bevacizumab for CNV from other causes than AMD and among them 11 patients suffered from AS. The CNV responded well to bevacizumab injections, however, as the authors stated, in between these eyes there was a high proportion of eyes that had previously undergone PDT. These results were in agreement with those reported by Donati and colleagues [66] who concluded that bevacizumab may be useful in the treatment of CNV due to AS. However, their results may have been compromised by the fact that all eyes had previously undergone PDT or laser photocoagulation. Three of six eyes were treated combining PDT and IV Avastin. Cases were followed up for 7-14 months. Five out of 6 eyes showed an improvement of BCVA and a slight reduction of leakage and size with FA. In a study by Tilleul and colleagues [67], 35 eyes of 27 patients were treated with repeated intravitreal ranibizumab injections (mean of 9.9 ± 7.2 injections, range 2-26) for a mean of 48.6 ± 17.1 months (range 8-66) for AS-associated CNV. Macular thickness had stabilized or decreased in 16 of 35 eyes (45.7%). At the last follow-up examination, on fluorescein angiography, no further leakage was observed in 27 of 35 eyes (77.1%). Martinez-Serrano and colleagues [68], despite initial anatomical and functional improvement, patients at the end of the follow-up had no visual improvement after a pro re nata regimen of antiangiogenic drugs. The amount of retreatments, number of recurrences, and time between intravitreal injections were similar to previous reports with shorter follow-up. The mean follow-up time was 53.8 ± 26.8 months. BCVA at baseline was: 1.001±0.62 logMAR; at the end of follow-up: 0.996 ± 0.56 logMAR (P=0.9). Central macular thickness at baseline was: 360.85 ± 173.82 μm; at the end of follow-up: 323.85±100.34 μm (P=0.6). Mean number of intravitreal angiogenic drugs: 6 ± 4.16 injections (range 4–15). Mean time between injections was 3.8 ± 2.7 months (range 1.9–5.8 months). A multicenter (n=23), observational study [69] was performed in France in 72 patients (98 eyes; mean age, 59.6 ± 8.3 years; 54.2% men) with CNV secondary to PXE who received at least one ranibizumab injection. The criterion for retreatment was based mainly on loss of VA, progression of CNV and angiographic leakage. CNV was primarily subfoveal or juxtafoveal (73.4%), and the initial mean VA was 64.6 ETDRS letters. On average, visual acuity of all eyes analyzed was relatively stable during the 2-year follow-up (62.3 letters vs 64.6 letters at the first injection). It was concluded that ranibizumab was able to significantly reduce the frequency of neovascularization relapses. Mimoun and colleagues [70] recommended three loading doses of ranibizumab followed by Pro Re Nata (PRN) regimen based on OCT and FA ctiteria for AS-associated CNV. There have been numerous reports on the treatment of refractive AS-associated CNV with intravitreal aflibercept [71, 72]. In a case report by Makein and colleagues [71], a 42-year-old female with AS-associated CNV underwent intensive treatment with ranibizumab without significant functional or anatomic change. After 3 loading doses of aflibercept, BCVA improved from 3/10 to 6/10, while OCT demonstrated resolution of the subretinal fluid. Gliem and colleagues [73] reported on the results of a 12-month prospective interventional clinical trial evaluating the use of intravitreal aflibercept for treatment of CNV secondary to AS in patients with PXE. Fifteen PXE patients with CNV received one initial intravitreal injection of 2 mg aflibercept. Further injections were based on CNV activity at monthly examinations. BCVA improved from 75.0 ± 10.8 to 79.3 ± 7.3 ETDRS letters at final visit (p = 0.083). Central retinal thickness (CRT) decreased from 317 ± 81 to 279 ± 51 μm (p = 0.004). The mean number of injections was 6.7 ± 2.6, and 5 participants had persistent or reactivated CNV activity at final visit. The Italian EYLEA-STRIE study [74] investigated the effect and the safety of intravitreal aflibercept in 20 patients (23 eyes) affected by CV secondary to AS. The patients received intravitreal 2 mg/0.05 mL aflibercept at the time of enrolment, followed by a pro-re-nata regimen for 48 weeks. At 6 month thereafter, BCVA stabilized and CRT decreased in study eyes. Eleven ocular non-serious adverse events and two serious adverse events were observed (one case of endophthalmitis and one case of acute gastritis were reported). As far as the safety profile of anti-VEGF agents is concerned, studies demonstrated no correlation of the treatment with postinjection intraocular pressure peaks, hyposphagmas, endophthalmitis, uveitis, intravitreal hemorrhage, lens trauma, retinal tear, or detachment [8, 70]. Savastano and colleagues [75] described the long-term effectiveness and tolerability of intravitreal VEGF inhibitor ranibizumab in a patient with PXE with bilateral macular CNV secondary to angioid streaks. As anti-vascular endothelial growth factor agents are associated with increased risk of systemic effects, particularly arterial thromboembolic events, following intravenous administration, the absence of serious thromboembolic or cardiovascular adverse events throughout the 6-year treatment period was particularly encouraging considering their patient’s high cardiovascular risk status. The visual prognosis in patients with CNV secondary to AS if untreated is poor and most treatment modalities, until recently, have failed to limit the devastating impact of CNV in central vision. However, as it is likely that anti-VEGF treatment to yield favorable results in the future, this approach warrants further research [9]. Conclusion AS are a disease of the fundus, usually bilateral and usually associated with a systemic condition (PXE, Paget’s disease, and etc.). Several diagnostic techniques (ophthalmoscopy, OCT, FA, and etc.) are helpful in establishing the diagnosis. Data is scarce with regard to the state of the choroid in AS. CNV is the most common complication leading to reduced vision. Intravitreal anti-VEGF administration is currently the most effective treatment for CNV.
References 1.Doyne RW. Choroidal and retinal changes. The results of blows on the eyes. Trans Ophthalmol Soc UK. 1889;9:128. 2.Knapp H. On the formation of dark angioid streaks as unusual metamorphosis of retinal hemorrhage. Arch Ophthalmol. 1892;26:289–92. 3.Kofler A. Beitrage zur Kenntnis der Angioid streaks. Arch Augenheilkd. 1917;82:134–49. 4.Bock Z. KIinik und Anatomie der gefassahnlichen Streifen im Augenhintergrund. Z Augenheilkd. 1938;95:1–50. 5.Hagedoorn A. Angioid streaks. Arch Ophthalmol. 1939;21:746–4. 935–65. 6.Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–92. 7.Tripathy K, Quint JM. Angioid Streaks (Knapp Streaks). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 June 01. 8.Chatziralli I, Saitakis G, Dimitriou E, Chatzirallis A, Stoungioti S, Theodossiadis G, Theodossiadis P. ANGIOID STREAKS: A Comprehensive Review From Pathophysiology to Treatment. Retina (Philadelphia, Pa.). 2019 Jan;39(1):1-11. 9.Georgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, Ladas I. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag. 2009 Feb;5(1):81-9. 10.Singman E, Doyle J. Angioid streaks are not a common feature of Ehlers Danlos Syndrome. JAMA Ophthalmology. 2018.137(3). 11.Brady AF, Demirdas S, Fournel-Gigleux S, et al. The Ehlers-Danlos syndromes, rare types. Am J Med Genet C Semin Med Genet. 2017;175(1):70-115. 12.Uitto J, Jiang Q, Varadi A, Bercovitch LG, Terry SF. Pseudoxanthoma Elasticum: diagnostic features, classification, and treatment options. Expert Opin Orphan Drugs. 2014;2:567–77. 13.Bercovitch L, Terry P. Pseudoxanthoma elasticum 2004. J Am Acad Dermatol. 2004;51:S13–S14. 14.Finger RP, Charbel Issa P, Ladewig MS, Gotting C, Szliska C, Scholl HP, Holz FG. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272–285. doi: 10.1016/j.survophthal.2008.12.006. 15.Mizutani Y, Nakayama T, Asai S, et al. ABCC6 mutation in patients with angioid streaks. Int J Biomed Sci. 2006;2:7–12. 16.Sato N, Nakayama T, Mizutani Y, Yuzawa M. Novel mutations of ABCC6 gene in Japanese patients with Angioid streaks. Biochem Biophys Res Commun. 2009;380:548–53. 17.Katagiri S, Negishi Y, Mizobuchi K, et al. ABCC6 gene analysis in 20 Japanese patients with angioid streaks revealing four frequent and two novel variants and pseudodominantin heritance. J Ophthalmol. 2017;2017:1079687. 18.Booij JC, Baas DC, Beisekeeva J et al. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. 19.19 Vit VV. [Pathology of the eye, ocular adnexa and orbit]. Vol. 2. Odesa:Astroprint; 2019. Russian. 20.Keith CG. Angioid streaks and pseudoxanthoma elasticum. Br J Ophthalmol. 1956;40:480–6. 21.Martinez-Serrano MG, Rodriguez-Reyes A, Guerrero-Naranjo JL, et al. Long-term follow-up of patients with choroidal neovascularization due to angioid streaks. Clin Ophthalmol. 2016;11:23–30. 22.Myung JS, Bhatnagar P, Spaide RF et al. Long-term outcomes of intravitreal antivascular endothelial growth factor therapy for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina. 2010;30:748–55. 23.Gal-Or O, Balaratnasingam C, Freund KB. Optical coherence tomography angiography findings of choroidal neovascularization in pseudoxanthoma elasticum. Int J Retina Vitreous. 2015;1:11. 24.Abusamak M, Lee AG, Berry S, Sadaka A. Angioid streaks. Medscape [Internet]. 2019 Jul 22. Available from: https://emedicine.medscape.com/article/1190444-overview#a2. 25.Ellabban AA, Tsujikawa A, Matsumoto A, Ogino K, Hangai M, Ooto S, Yamashiro K, Akiba M, Yoshimura N. Macular choroidal thickness and volume in eyes with angioid streaks measured by swept source optical coherence tomography. Am J Ophthalmol. 2012 Jun;153(6):1133-43.e1. 26.Roach ES, Islam MP. Pseudoxanthoma elasticum. Handb Clin Neurol. 2015;132:215-21. 27.Gandorfer A, Ulbig M, Bechmann M, et al. Retinal telangiectasis and angioid streaks. Br J Ophthalmol. 2000;84:1327–8. 28.Baillif-Gostoli S, Quaranta-El Maftouhi M, Mauget-FaysseM. Polypoidal choroidal vasculopathy in a patient with angioid streaks secondary to pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2010;248:1845–8. 29.Cebeci Z, Bayraktar S, Oray M, Kir N. Silent polypoidalchoroidal vasculopathy in a patient with angioid streaks. Arq Bras Oftalmol. 2016;79:200–1. 30.Shields JA, Federman JL, Tomer TL, Annesley WH., Jr Angioid streaks. I. Ophthalmoscopic variations and diagnostic problems. Br J Ophthalmol. 1975;59:257–66. 31.Mansour AM, Ansari NH, Shields JA, Annesley WH, Jr, Cronin CM, Stock EL. Evolution of angioid streaks. Ophthalmologica. 1993;207:57–61. 32.Mansour AM, Shields JA, Annesley WH, Jr, el-Baba F, Tasman W, Tomer TL. Macular degeneration in angioid streaks. Ophthalmologica. 1988;197:36–41. 33.Al-Rashaed S, Arevalo JF. Long-term follow-up of choroidal neovascularization secondary to angioid streaks: case series and literature review. Clin Ophthalmol. 2012;6:1029–34. 34.Savko VV, Naritsyna NI, Chechin PP, Konovalova NV, Serebrina TM, Novik AIa. [Grönblad–Strandberg syndrome: clinical features, diagnosis and treatment]. Oftalmol Zh. 2009;4:82-5. Russian. 35.Smith JL, Gass JD, Justice JJr. Fluorescein fundus photography of angioid streaks. Br J Ophthalmol. 1964;48:517–21. 36.Sawa M, Ober MD, Freund KB, Spaide RF. Fundus autofluorescence in patients with pseudoxanthoma elasticum. Ophthalmology. 2006113820e1–2. 37.Finger RP, Charbel Issa P, Ladewig M, et al. Fundus autofluorescence in Pseudoxanthoma elasticum. Retina. 2009;29:1496–1505. 38.De Zaeytijd J, Vanakker OM, Coucke PJ, et al. Added value of infrared, red-free and autofluorescence fundus imaging in pseudoxanthoma elasticum. Br J Ophthalmol. 2010;94:479–86. 39.Lafaut BA, Leys AM, Scassellati-Sforzolini B et al. Comparison of fluorescein and indocyanine green angiography in angioid streaks. Graefes Arch Clin Exp Ophthalmol.1998;236:346–53. 40.Peretyagina DO, Ulyanova NA. SS-OCT-derived morphometric changes in the choroid in patients with age-related macular degeneration. Journal of Ophthalmology (Ukraine). 2019;6:63-9. http://doi.org/10.31288/oftalmolzh201966369. 41.Iegorova KS, Znamenska MA, Guk MO, Mumliev AO. Early signs of primary compressive optic atrophy evidenced by OCT in patients with basal brain tumors. Journal of Ophthalmology (Ukraine). 2020;1:35-9. 42.Marchese A, Parravano M, Rabiolo A, Carnevali A, Corbelli E, Cicinelli MV, et al. Optical coherence tomography analysis of evolution of Bruch`s membrane features in angioid streaks. Eye (Lond). 2017 Nov; 31(11): 1600-5. 43.Chapron T, Mimoun G, Miere A, Srour M, Ameen El A, Semoun O, Souied EN. Optical coherence tomography angiography features of choroidal neovascularization secondary to angioid streaks. Eye (Lond). 2019 Mar; 33(3): 385–91. 44.Orly Gal-Or, Chandrakumar Balaratnasingam, Bailey Freund K. Optical coherence tomography angiography findings of choroidal neovascularization in pseudoxanthoma elasticum. Int J Retina Vitreous. 2015; 1:11. 45.Mansour AM; Ansari NH; Shields JA; Annesley WH; Cronin CM; Stock E. Evolution of angioid streaks. Ophthalmologica. 1993; 207(2):57-61. 46.Clarkson JG, Altman RD. Angioid streaks. Surv Ophthalmol. 1982;26:235–46. 47.Singerman LJ, Hatem G. Laser treatment of choroidal neovascular membranes in angioid streaks. Retina. 1981;1:75–83. 48.Meislik J, Neldner K, Reeve EB, Ellis PP. Laser treatment in maculopathy of pseudoxanthoma elasticum. Can J Ophthalmol. 1978;13:210–2. 49.Deutman AF, Kovacs B. Argon laser treatment in complications of angioid streaks. Am J Ophthalmol. 1979;88:12–7. 50.Gelisken O, Hendrikse F, Deutman AF. A long-term follow-up study of laser coagulation of neovascular membranes in angioid streaks. Am J Ophthalmol. 1988;105:299–303. 51.Aras C, Baserer T, Yolar M, et al. Two cases of choroidal neovascularization treated with transpupillary thermotherapy in angioid streaks. Retina. 2004;24:801–803. 52.Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1993;231:635–41. 53.Roth DB, Estafanous M, Lewis H. Macular translocation for subfoveal choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2001;131:390–2. 54.Fujii GY, Humayun MS, Pieramici DJ, Schachat AP, Au Eong KG, E de Juan Jr. Initial experience of inferior limited macular translocation for subfoveal choroidal neovascularization resulting from causes other than age-related macular degeneration. Am J Ophthalmol. 2001;131:90–100. 55.Sickenberg M, Schmidt-Erfurth U, Miller JW, et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch Ophthalmol. 2000;118:327–36. 56.Karacorlu M, Karacorlu S, Ozdemir H, Mat C. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol. 2002;134:360–6. 57.Shaikh S, Ruby AJ, Williams GA. Photodynamic therapy using verteporfin for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2003;135:1–6. 58.Menchini U, Virgili G, Introini U, et al. Outcome of choroidal neovascularization in angioid streaks after photodynamic therapy. Retina. 2004;24:763–71. 59.Arias L, Pujol O, Rubio M, Caminal J. Long-term results of photo-dynamic therapy for the treatment of choroidal neovascularization secondary to angioid streaks. Graefes Arch Clin Exp Ophthalmol. 2006 Jun;244(6):753-7. 60.Ladas ID, Georgalas I, Rouvas AA, Gotsis S, Karagiannis DA, Moschos M. Photodynamic therapy with verteporfin of choroidal neovascularization in angioid streaks: conventional versus early retreatment. Eur J Ophthalmol. 2005;15:69–73. 61.Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313-1330. 62.Korol AR, Zadorozhnyy OS, Naumenko VO, Kustryn TB, Pasyechnikova NV. Intravitreal aflibercept for the treatment of choroidal neovascularization associated with pathologic myopia: a pilot study. Clin Ophthalmol. 2016;10:2223-9. 63.Pasyechnikova NV, Naumenko VO, Korol AR, Zadorozhnyy OS, Kustryn TB, Henrich PB. Intravitreal ranibizumab for the treatment of choroidal neovascularizations associated with pathologic myopia: a prospective study. Ophthalmologica. 2015;233(1):2-7. 64.Wecke T, Knop C, Schreiber W, Behrens-Baumann W. Intraocular injections of bevacizumab in rare indications – two cases. Ophthalmologe. 2009 May; 106(5):435-42. doi: 10.1007/s00347-008-1782-3. 65.Chang LK, Spaide RF, Brue C, Freund KB, Klancnik JM, Jr, Slakter JS. Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration. Arch Ophthalmol. 2008;126:941–5. 66.Donati MC, Virgili G, Bini A, et al. Intravitreal bevacizumab (Avastin) for choroidal neovascularization in angioid streaks: A case series. Ophthalmologica. 2008;223:24–7. 67.Tilleul J, Mimoun G, Querques G, Puche N, Zerbib J, Lalloum F, Srour M, Souied EH. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks: Four-Year Follow-up. Retina. 2016; 36(3):483-91. 68.Martinez-Serrano MG, Rodriguez-Reyes A, Guerrero-Naranjo JL, Salcedo-Villanueva G, Fromow-Guerra J, García-Aguirre G, et al. Long-term follow-up of patients with choroidal neovascularization due to angioid streaks. Clin Ophthalmol. 2017; 11:23-30. 69.Ebran JM, Mimoun G, Cohen SY, Grenet T, Donati A, Jean-Pastor MJ, Ponthieux A, Bouchet C. Treatment with ranibizumab for choroidal neovascularization secondary to a pseudoxanthoma elasticum: Results of the French observational study PiXEL. J Fr Ophtalmol. 2016; 39(4):370-5. 70.Mimoun G, Tilleul J, Leys A, et al. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2010;150:692–700. 71.Makri OE, Tsapardoni FN, Plotas P, Pallikari A, Georgakopoulos CD. Intravitreal aflibercept for choroidal neovascularization secondary to angioid streaks in an on-responderto intravitreal ranibizumab. Int Med Case Rep J. 2018; 11:229-31. 72.Esen E, Sizmaz S, Demircan N. Intravitreal aflibercept for management of subfoveal choroidal neovascularization secondary to angioid streaks. Indian J Ophthalmol. 2015; 63(7):616-8. 73.Gliem M, Birtel J, Herrman P, Fimmers R, Berger M, Coch C, Wingen A, Holz FG, Issa PC. Aflibercept for choroidal neovascularizations secondary to pseudoxanthoma elasticum: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2020; 258:311-8. 74.Parodi MB, Cicinelli MV, Marchese A, Giuffre C, Francesco V, Staurenghi G, Varano M, Bandello F. Intravitreal aflibercept for management of choroidal neovascularization secondary to angioid streaks: The Italian EYLEA-STRIE study. Eur J Ophtalmol. 2020 Jun 2;1120672120928305. 75.Savastano MC, Minnella AM, Zinzanella G, et al. Successful long-term management of choroidal neovascularization secondary to angioid streaks in a patient with pseudoxanthoma elasticum: a case report. J Med Case Rep. 2014;8:458. Funding: This study did not receive any funding support.
|