J.ophthalmol.(Ukraine).2021;2:23-30.
|
http://doi.org/10.31288/oftalmolzh202122330 Received: 04 February 2020; Published on-line: 19 April 2021 Comparing the efficacy of monofocal and multicfocal IOLs in phacoemulsification of cataract in eyes with high myopia N. G. Zavgorodnia1,3, N. V. Mykhailenko1,3, L. E. Sarzhevska1, T. S. Zavgorodnia2,3, A. S. Sarzhevskii1,3 1 Zaporizhzhia State Medical University, Zaporizhzhia (Ukraine) 2 Shupyk National Medical Academy of Postgraduate Education, Kyiv (Ukraine) 3 VISUS clinic, Zaporizhzhia (Ukraine) E-mail: doc.mikhaylenko89@gmail.com TO CITE THIS ARTICLE: Zavgorodnia NG, Mykhailenko NV, Sarzhevska LE, Zavgorodnia TS, Sarzhevskii AS. Comparing the efficacy of monofocal and multicfocal IOLs in phacoemulsification of cataract in eyes with high myopia. J.ophthalmol.(Ukraine).2021;2:23-30. http://doi.org/10.31288/oftalmolzh202122330 Background: High myopia is a factor that complicates and may affect the visual outcome of phacoemulsification of cataract. There is no agreement among ophthalmologists as to whether it is appropriate to implant a multifocal intraocular lens (IOL) in high myopia. Such doubts are caused by frequent complications in the fundus which cannot be found before surgery, leading to the impossibility of an accurate postoperative visual prognosis. However, the patient’s desire to improve vision does not allow abandoning the idea of implanting a multifocal lens in eyes with high myopia. Purpose: To compare treatment outcomes among eyes with high myopia which underwent phacoemulsification with implantation of monofocal versus multifocal IOLs. Material and Methods: We reviewed the visual outcomes of 55 patients (93 eyes) with high myopia who had undergone phacoemulsification and IOL implantation. Study eyes were divided into two groups depending on the type of IOL implanted. Group A included 22 patients (38 eyes) with a multifocal IOL implanted. In this group, there were 11 women (55%) and 9 men (45%), and patient age ranged from 29 years to 73 years (mean age, 52 ± 2.9 years). Group B included 32 patients (55 eyes) with a monofocal IOL implanted. In this group, there were 18 women (51.4%) and 16 men (48.6%), and patient aged ranged from 32 years to 78 years (mean age, 58 ± 2.1 years). Preoperatively, patients underwent a routine eye examination which included automated refraction, visual acuity, perimetry, Amsler test, phosphene test, tonometry, biomicroscopy, direct ophthalmoscopy, and, if possible, retinal examination using a Goldmann lens. In addition, they underwent a special examination for the patients being prepared for phaco plus IOL implantation which included ultrasound A- and B-scanning (Alcon; USA), optical biometry (IOL Master 700 - Carl Zeiss Meditec AG, Germany), and endothelial microscopy (SP-3000P, Topcon Corporation, Japan). The Haigis and SRK-T formulas were used for IOL calculation. Results: At one month after phacoemulsification, eyes in both groups showed improved visual acuity. In the eyes of group A, mean uncorrected visual acuity (UCVA) increased by 76% to 0.8 ± 0.03 and mean best-corrected visual acuity (BCVA), by 86% to 0.9±0.02, whereas mean Sph and mean Cyl values were -0.06 ± 0.08 D and -0.59 ± 0.15 D, respectively. In the eyes of group B, UCVA increased by 49% to 0.55 ± 0.02 and mean BCVA, by 78% to 0.84±0.02, whereas mean Sph and mean Cyl values were -0.85 ± 0.11 D and -0.94 ± 0.12 D, respectively. These results maintained for six months, and the difference between values at month 1 and month 6 was not statistically significant (p > 0.05) for all cases. After phacoemulsification and IOL implantation, in the eyes of group A, mean spherical component decreased by 12.88 ± 0.2 D to -0.22±0.11 D, whereas mean cylindrical component decreased by 1.0 ± 0.1 D to -0.8 ± 0.1 D as assessed by autoreftactometry. In addition, at one month, in the eyes of group B, mean spherical component decreased by 12.63 ± 0.12 D to -0.84 ± 0.02 D, and approached a target value, whereas a change in a cylindrical component was not statistically significant. The eyes with an implanted multifocal IOL were found to be well adapted to near work and showed a mean near vision acuity of 0.9 ± 0.1 versus 0.6 ± 0.1 for the eyes with an implanted monofocal IOL. Aberrations were significantly less in group A than in group B. Conclusion: First, among the eyes with high myopia, the percentage increase in uncorrected visual acuity was larger in those with an implanted multifocal IOL (76%) than in those with an implanted monofocal IOL (49%), and the difference was significant (р < 0.05). Second, the increase in near visual acuity was by 33% larger in those with an implanted multifocal IOL than in those with an implanted monofocal IOL, which significantly improved their quality of life, enabling them to quit wearing their glasses. Finally, our findings demonstrate that high myopia is not a contraindication for implanting a multifocal IOL. Keywords: High myopia, phacoemulsification of cataracts, multifocal IOL, monofocal IOL
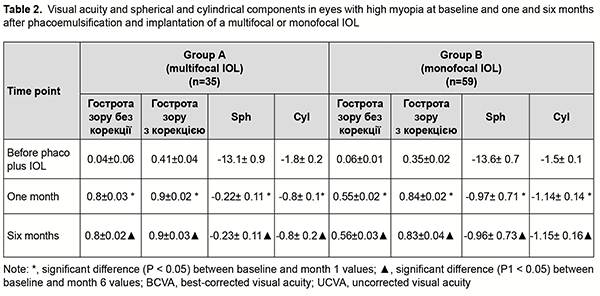
Introduction Cataract phacoemulsification has become the most common type of cataract surgery. In the case of cataract phacoemulsification, the risk of intra- and post-operative complications is reduced because the ocular cavity is almost closed during surgical manipulations, a small self-sealing incision is used and reliable intracapsular fixation of the intraocular lens (IOL) can be used [1]. Myopes comprise 10-25% of patients presenting with cataract. Thinning and extension of the ocular coats and lens ligaments, destruction and liquefaction of the vitreous, and impaired ocular hemodynamics and hydrodynamics are characteristic for myopic eyes (especially, eyes with high myopia) [1], and may be risk factors for phacoemulsification. Myopic eyes have a thicker but substantially weaker posterior lens capsule, and weaker ciliary zonules, compared to emmetropic eyes [2]. These facts may explain a high risk of posterior capsular rupture and rupture of the lens apparatus in cataract surgery during instrument contact or excessive build-up of pressure inside the capsular bag (hydrodissection and hydrodelineation), lens polishing, and aspiration of equatorial cortical lens material. Compromised capsular bag integrity and hydrodynamic shock waves hitting the anterior vitreous have been reported to increase the likelihood of postoperative proliferation in the vitreous and subsequent traction retinal detachment [3]. A variety of potential complications during phaco in myopic eyes make the choice of IOL type very important. Numerous IOLs from various manufacturers and with various characteristics are currently available, and the surgeon can select the model based on his/her clinical experience and patient’s preferences [4]. A monofocal IOL provides clear vision at one particular distance and is a preferred type of IOL for implantation in myopic eyes for most phaco surgeons. Myopic refraction is left in such eyes; this provides the patient with a usual mode of vision, but not with maximum visual acuity at distance. With advent of multifocal IOLs to the Ukrainian market, the question arose whether it is reasonable to use them in cataractous eyes with high myopia. Previous studies on multifocal IOLs have determined that the success of refractive correction with a multifocal IOL requires achieving a distance visual acuity of 0.7 or better and a near visual acuity of 0.5 or better [5]. More than 85% of patients did not require glasses after they received multifocal IOLs [5]. It should be taken in account that the IOLs of this type vary in design and some of them require a well functioning pupil and should be aligned correctly to implement their bifocal functions fully [6-9]. This study was conducted because there is no agreement among cataract surgeons as to whether it is feasible to implant multifocal IOLs in cataract phacoemulsification in eyes with high myopia. The purpose of this study was to compare treatment outcomes among eyes with high myopia which underwent phacoemulsification with implantation of monofocal versus multifocal IOLs. Material and Methods We reviewed treatment outcomes of phacoemulsification with IOL implantation for 55 patients (93 eyes) with cataract and high myopia. Study eyes were divided into two groups depending on the type of IOL implanted. Group A included 22 patients (38 eyes) with a multifocal IOL implanted. In this group, there were 11 women (55%) and 9 men (45%), and patient age ranged from 29 years to 73 years (mean age, 52 ± 2.9 years). Group B included 32 patients (55 eyes) with a monofocal IOL implanted. In this group, there were 18 women (51.4%) and 16 men (48.6%), and patient aged ranged from 32 years to 78 years (mean age, 58 ± 2.1 years). That is, the groups were comparable with regard to age and gender. Preoperatively, patients underwent a routine eye examination which included automated refraction, visual acuity, perimetry, Amsler test, phosphene test, tonometry, biomicroscopy, direct ophthalmoscopy, and, if possible, retinal examination using a Goldmann lens. In addition, they underwent a special examination for the patients being prepared for phaco plus IOL implantation which included ultrasound A- and B-scanning (Alcon; USA), optical biometry (IOL Master 700 - Carl Zeiss Meditec AG, Jena, Germany), and endothelial microscopy (SP-3000P, Topcon Corporation, Tokyo, Japan). The Haigis and SRK-T formulas were used for IOL calculation. The contrast sensitivity was determined as per the method of Birich et al [10], using the matrix contrast optotype chart TB-1, where, in a way similar to the Golovin-Sivtsev chart, modified Landolt rings are arranged top down in a particular order according to the international vision assessment standards. The contrast of modified Landolt rings decreases along a chart line left to right from 1 to 0.2, which corresponds to contrast sensitivity of 1 to 5. The separating power was recorded for the number located in the vertical row of numbers against the particular chart line. The contrast sensitivity was assessed using the table developed by the authors, with a value of 5 conforming to excellent contrast sensitivity; 3, to good; 2.5, to satisfactory; 2, to low; and 1.5-1, to poor contrast sensitivity. Of the 38 eyes which received multifocal IOLs in group A, 11 received AT Lisa tri 839 (Carl Zeiss, Germany); 16, MP Lisa 809M (Carl Zeiss); seven, MULTIDIFF 605 (Appasamy Associates, India); one, AT LARA 829MP (Carl Zeiss); and three, AcrySof® IQ ReSTOR® (Alcon, USA). Of the 55 eyes which received monofocal IOLs in group B, 19 received CT LUCIA 601P (Carl Zeiss); 18, CT ASPHINA 409MV (Carl Zeiss); eight, NASPRO BBY (Appasamy Associates); three, SL-902 (US-optics, Ukraine); two, ACRYFOLD 601Y (Appasamy Associates); one, Supraphob BBY (Appasamy Ocular Devices (P) LTD, India); one, Acry sof IQ SN 60 WF (Alcon); and two, Eyeol LW5752R (Eyeol UK, UK). Cataract surgeries were performed by three surgeons with an individual induced astigmatism not exceeding 0.5D. No complications were observed during or after surgery. Follow-up duration was 6 months. All studies and surgeries were performed at the VISUS clinic, the clinical setting of the Department of Ophthalmology at the Zaporizhzhia State Medical University. Informed consent for personal data processing was obtained from all participants of the study. Statistical analyses were conducted using Statistica 10, BiostatPro 6, and Microsoft Excel 2016 software. Shapiro–Wilk W test was used to test normality. Data are presented as mean (M) and standard error of mean (m). Student t test was used for paired series if the data fit for normal distribution. The level of significance p < 0.05 was assumed. Results Groups A and B were found to be comparable not only with regard to age and gender, but also with regard to characteristics of myopia (Table 1). Mean spherical equivalent (Sph) and mean cylindrical equivalent (Cyl) were -13.1±0.98 D and -1.8 ± 0.29 D, respectively, for group A, and -13.6 ± 0.7 D and -1.5 ± 0.14 D, respectively, for group B. Mean axial length of the eye was 28.53 ± 0.31 mm for group A and 28.52 ± 0.24 mm for group B, but the difference was not statistically significant (р > 0.05) for all cases. Mean lens size was 4.41 ± 0.06 mm for group A and 4.35 ± 0.07 mm for group B, but the difference was also not statistically significant (р > 0.05).
Groups A and B were also comparable with regard to pre-treatment visual acuity. Before surgery, mean uncorrected visual acuity was 0.04 ± 0.006, mean Sph, -12.2 ± 0.8 D, mean Cyl, -1.01 ± 0.14 D, and mean best-corrected visual acuity (BCVA), 0.41 ± 0.04, for group A, and 0.06 ± 0.01, -13 ± 0.6 D, -1.12 ± 0.13 D and 0.35 ± 0.02, respectively, for group B (р > 0.05). It should be noted that the power of a monofocal IOL for a high myope was calculated so to achieve some small residual myopia for better postoperative adaptation. An amount of target residual myopia was not more than 0.5 D for an eye with a multifocal IOL, and -1.0 D for an eye with a monofocal IOL. At one month after phacoemulsification, eyes in both groups showed improved visual acuity (Table 2). In the eyes of group A, mean UCVA increased by 76% to 0.8 ± 0.03 and mean BCVA, by 86% to 0.9±0.02, whereas mean Sph and mean Cyl values were -0.06 ± 0.08 D and -0.59 ± 0.15 D, respectively.
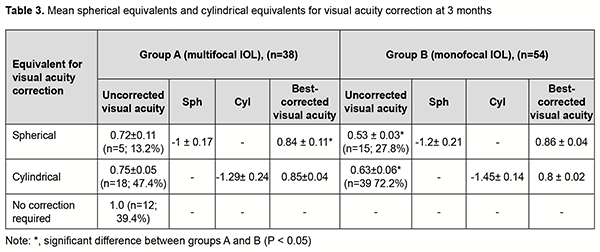
In the eyes of group B, mean UCVA increased by 49% to 0.55 ± 0.02 and mean BCVA, by 78% to 0.84±0.02, whereas mean Sph and mean Cyl values were -0.85 ± 0.11 D and -0.94 ± 0.12 D, respectively. These results maintained for six months, and the difference between values at month 1 and month 6 was not statistically significant (p > 0.05) for all cases. After phacoemulsification and IOL implantation, in the eyes of group A, mean spherical component decreased by 12.88 ± 0.2 D to -0.22±0.11 D, whereas mean cylindrical component decreased by 1.0 ± 0.1 D to -0.8 ± 0.1 D as assessed by autoreftactometry. In addition, at one month, in the eyes of group B, mean spherical component decreased by 12.63 ± 0.12 D to -0.84 ± 0.02 D, and approached a target value, whereas a change in a cylindrical component was not statistically significant. It is noteworthy that most patients in both groups did not use cylindrical correction even when it was beneficial for visual acuity. Table 3 presents the data on the cylindrical component of refraction and its influence on the visual acuity after implantation of a monofocal or multifocal IOL in eyes with high, uncomplicated myopia.
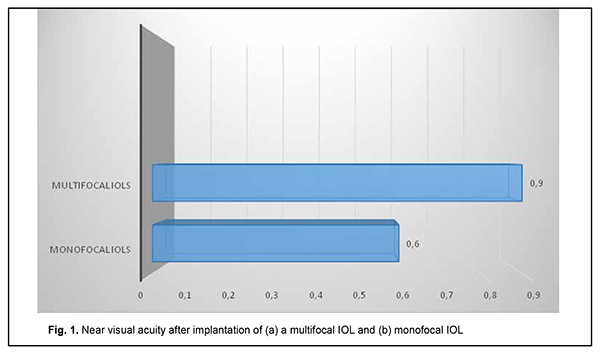
As it can be seen from table 3, a target refraction (-0.75 ± 0.25 D) was achieved in patients of both groups. At 3 months after surgery, in group A, a spherical correction of -1 ± 0.17 D improved visual acuity from 0.72 ± 0.11 to 0.84 ± 0.11 in 5 eyes (13.2%). In addition, 18 eyes (47.3%) required a cylindrical correction of -1.29 D, and 100 percent visual acuity was achieved without any correction in 12 eyes (39.4%). In eyes of group B (monofocal IOL), although a target refraction (-0.75 ± 0.25 D) was achieved, the visual acuity at distance was significantly lower than in eyes of group A (multifocal IOL). Spherical correction improved visual acuity in 15 eyes (27.7%), and cylindrical correction, in 39 eyes (72.2%). Preoperatively, patients with corneal astigmatism of both groups refused to have a toric IOL implanted. They could receive excimer laser correction of residual astigmatism using the bioptics approach in the future, if required. The eyes with an implanted multifocal IOL were found to be well adapted to near work and showed a mean near vision acuity of 0.9 ± 0.1 versus 0.6 ± 0.1 for the eyes with an implanted monofocal IOL (Fig. 1).
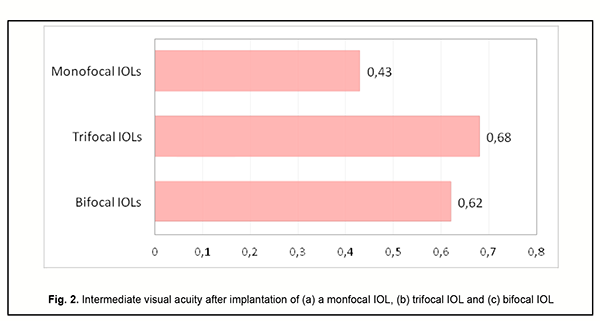
Although the amount of difference in near vision acuity between the two groups was substantial, no patient in either group wore glasses for work during the postoperative period. This indicated that myopic eyes became well adapted for near work. Patients with implanted monofocal IOLs were generally satisfied with their treatment outcomes, but had to wear their glasses for distance which caused some visual discomfort. Eyes with a trifocal IOL (n=13) showed the best intermediate visual acuity (0.68 ± 0.11), followed by eyes with a bifocal IOL (n=25) (0.62 ± 0.11) and eyes with a monofocal IOL (n=54) (0.43 ± 0.09) (Fig. 2).
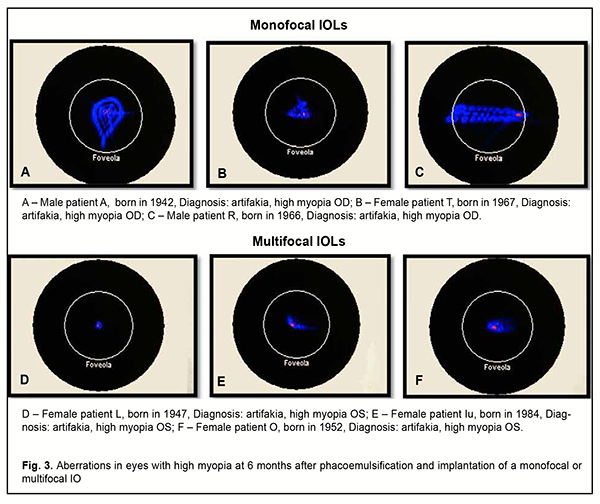
No eye showed excellent contrast sensitivity, but mean contrast sensitivity was 0.7 ± 0.01 higher in eyes with a monofocal IOL (3.5 ± 0.12; good contrast sensitivity) than in eyes with a multifocal IOL (2.98 ± 0.13; satisfactory of good contrast sensitivity). However, patients in both groups were generally satisfied with their treatment outcomes, and found the visual acuity at distance and the opportunity to read without glasses to be more important to be more important than the contrast sensitivity. Our wavefront analysis of study eyes at six months showed that, under mesopic conditions, the eyes with an implanted multifocal IOL had less total spherical aberration compared to those with an implanted monofocal IOL. The eyes with an implanted monofocal IOL varied in the extent of aberration and shower greater area and decentration of aberrations, which affected their visual acuity (Fig. 3).
Discussion Although there have been numerous reports on the advantages of implanting a multifocal IOL after phacoemulsification, the question of selecting an IOL for eyes with high myopia is still open. We found phacoemulsification with IOL implantation to improve visual acuity in eyes with high myopia in both groups. However, implantation of a multifocal IOL was found to result in good visual acuity at near and far distances in high myopes. Compared to those with an implanted monofocal IOL, these patients are better adapted to new conditions of vision, which significantly improves their quality of life. Our findings are in agreement with those of others. Morozova and Kerimov [11] studied patient satisfaction with treatment outcomes, independence from spectacles and dysphotopsia occurring after implantation of a multifocal IOL on the basis of personal experience and literature review. They noted that, although there is no ideal design for a multifocal IOL, at the later follow-up time points, there was a high level of subjective satisfaction of those patients who underwent implantation of a multifocal IOL, with a significant reduction in the risk and severity of light-associated events (halo, flicker, etc.) in the postoperative period. Among patients experiencing these events, tolerance to dysphotopsia commonly developed before month 6 after surgery. In addition, uncorrected near visual acuity and contrast sensitivity were found to be improved with time, although there was no change in refractive error. Those authors believe that adaptation to an implanted multifocal IOL is associated with neuroadaptation of the whole visual system to it, but this process does not require any effort from the patient. Tur and colleagues [12] reviewed the medical records of 405 patients (668 eyes) with high myopia who had undergone implantation of a multifocal IOL. They noted that implantation of a multifocal IOL in eyes with high myopia resulted in an improvement in visual acuity to 1.0 even in the presence of refractive amblyopia, with maintenance of good visual acuity at near. Tur and colleagues [12] believe that this improvement in vision was associated with an increase in the area of the image falling onto the retina as well as a decrease in optical aberrations. Our wavefront analysis of eyes which underwent phacoemulsification showed that, the eyes with an implanted multifocal IOL had less number and area of total spherical aberration compared to those with an implanted monofocal IOL. In the current study, patients of both groups could read without glasses, but the eyes with an implanted multifocal IOL had significantly better visual acuity both at near and far distances compared to those with an implanted monofocal IOL. It is likely that the lower visual acuity at far distances in the eyes with an implanted monofocal IOL was associated with an amount of target residual myopia for an eye with a monofocal IOL (-1.0 D), but, nevertheless, this approach enabled them to feel comfortable under the usual conditions. The authors make the following conclusions. First, among the eyes with high myopia, the percentage increase in uncorrected visual acuity was larger in those with an implanted multifocal IOL (76%) than in those with an implanted monofocal IOL (49%), and the difference was significant (р < 0.05). Second, the increase in near visual acuity was by 33% larger in those with an implanted multifocal IOL than in those with an implanted monofocal IOL, which significantly improved their quality of life, enabling them to quit wearing their glasses. Finally, our findings demonstrate that high myopia is not a contraindication for implanting a multifocal IOL.
References 1.Resnikova EV. [Phacoemulsification in eyes with high myopia]. [Cand Sc (Med) Thesis]. Moscow: Helmholtz Research Institute for Eye Diseases; 2005. Russian. Available at: https://www.dissercat.com/content/fakoemulsifikatsiya-katarakty-pri-bliz... 2.Sheu SJ, Ger LP, Ho WL. Late increased risk of retinal detachment after cataract extraction. Am J Ophthalmol. 2010 Jan;149(1):113-9. 3.Tajunisah I, Reddy SC. Dropped nucleus following phacoemulsification cataract surgery. Med J Malaysia. 2007 Dec;62 (5):364-7. 4.Shukhaev SV, Matveeva AV, Kirillova OV, Zagorulko AM. [Comparative evaluation of target refraction between three monofocal flexible intraocular lenses]. Fyodorov Journal of Ophthalmic Surgery. 2018;(1):53-58. Russian. 5.Malyugin BE, Morozova TA. [A review of historical aspects and modern trends in multifocal intraocular correction]. Oftalmokhirurgiia. 2004;(3):23-9. Russian. 6.Dikovskaia MA, Iskakov IA. [Positioning MIOL-Akkord refractive and diffractive IOL in the capsular bag during implantation of the intracapsular ring]. In: [Advanced technologies in ophthalmology: A collection of science papers]. Krasnodar; 2008. p.36-38. Russian. 7.Iskakov IA, Druzhinin IB, Morozova IM, et al. [Degree of pupil independence in various designs of refractive and diffractive IOLs]. In: [Ophthalmology of the Black Sea countries: A collection of science papers]. Krasnodar; 2006. p.114-7. Russian. 8.Alvarez-Rementeria L, Montes-Mico R. Pseudoaccomodating intraocular lens implantation in patient with irregular nonreactive puppies. J Cataract Refract Surg. 2007 Oct;33(10):1823-5. 9.Hayashi K, Hayashi H, Nakao F, Hayashi F. Correlation between Pupillary Size and Intraocular Lens Decentration and Visual Acuity of a Zonal-progressive Multifocal IOL and a Monofocal IOL. Ophthalmology. 2001 Nov;108(11):2011-7. 10.Birich TA, Fedorov IuG, Chekina AIu, Motornyi VV. [Instructions on the fast approach for determining the contrast sensitivity of the eye with the use of the contrast optotype chart (patent No. 9853)]. Minsk; 2008. Russian. 11.Morozova TA, Kerimov TZ. [Modern Approaches to Dysphotopsia Analysis, Assessmentof Patient Satisfaction and Spectacle Independence after Multifocal Intraocular Correction: Review]. 12.VESTNIK RAMN. 2017; 72(5):355–64. Russian. 13.Tur EV, Pozdeieva VA, Sorokina IA, Boiko MV. [Refractive outcomes of implantation of a multifocal IOL in patients with high myopia and amblyopia]. Sovremennyie tekhnologii v oftalmologii. 2018;5:243-5. Russian.
Funding: This study did not receive any funding support. Conflict of interest: No conflict of interest to declare
|