J.ophthalmol.(Ukraine).2021;2:16-22.
|
http://doi.org/10.31288/oftalmolzh202121622 Received: 16 December 2020; Published on-line: 19 April 2021 Transcription factor NF-kB in the prognosis of outcomes for patients undergoing repeat keratoplasty Komakh Y. A.1, Borzenok S. A.1, Radygina T. V.2, Kuptsova D. G.2, Petrichuk S. V.2 1 Fyodorov Eye Microsurgery Federal State Institution of the Russian Ministry of Health; Moscow (Russian Federation) 2 National Medical Research Center for Children’s Health of the Russian Ministry of Health; Moscow (Russian Federation) E-mail: komakh@yandex.ru TO CITE THIS ARTICLE: Komakh YA, Borzenok SA, Radygina TV, Kuptsova DG, Petrichuk SV. Transcription factor NF-kB in the prognosis of outcomes for patients undergoing repeat keratoplasty. J.ophthalmol.(Ukraine).2021;2:16-22.http://doi.org/10.31288/oftalmolzh202121622 Background: Obtaining a clear graft after repeat keratoplasty is still a challenging task for corneal transplant surgeons. Corneal transplant rejection rate is significantly increased in repeat keratoplasty compared with first keratoplasty. Activation of nuclear factor kappa B (NF-kB) plays a key role in the pathogenesis of rejection of transplants of various organs and tissues, primarily due to production of proinflammatory cytokines (TNF, IL-1, etc.). Purpose: To assess the role of transcription factor NF-kB in lymphocyte subsets in the prognosis of outcomes for patients undergoing repeat keratoplasty. Material and Methods: Forty-six patients (46 eyes) who underwent repeat keratoplasty were included. Of these, 30 patients (group 1) developed an opacified graft, and 16 (group 2) obtained a clear graft. Patient age ranged from 32 to 88 years. Imaging flow cytometry (ImageStream Mark II – AMNIS) and Amnis NF-kB Translocation Kits (ACS10000, Millipore, Sigma) were used to assess percentages of the cells with translocation of the NF-kB p65 subunit for lymphocyte subsets. Statistical analyses were conducted using Statistica 13.0 (StatSoft, Tulsa, OK, USA) software. The Mann–Whitney test was used to assess significance of differences. Results: Percentages of the cells with translocation of the NF-kB p65 subunit were higher in group 1 compared with group 2, particularly, for T-helpers (26.5[17; 29] vs 12.2[11;21]; p=0.003), cytotoxic T-cells (24.0[17;28] vs 11.5[7;15]; p=0.006), NK cells (45.0[34;53] vs 18.2[16;39]; p=0.014), Th17 cells (23.9[18;31] vs 14.3[12;21]; p=0.002), regulatory T cells (30.4[21;34] vs 17.4[16;22]; p=0.001) and activated T helpers (21.918;28] vs 11.4[11;17]; p=0.002). Conclusion: There were substantially increased percentages of activated cells in lymphocyte subsets (with the most pronounced increase for NK cells) of patients who developed an opacified graft compared to those who obtained a clear graft following re-keratoplasty. Assessment of percentages of the cells with translocation of the NF-kB p65 subunit for lymphocyte subsets provides valuable information for predicting graft rejection following re-keratoplasty. Keywords: keratoplasty, graft opacification, immunophenotyping, transcription factor NF-kB
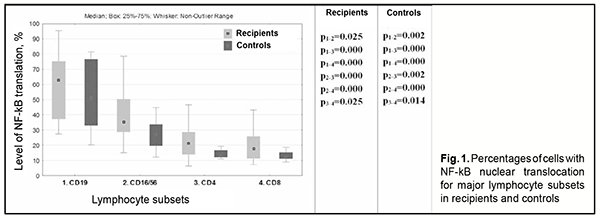
Introduction Corneal transplantation is the most commonly performed transplant surgery in the world today. In patients with high risk of corneal graft rejection, the success rate at 10 years is as low as 35% [1]. Moreover, corneal graft rejection rate is significantly increased in repeat keratoplasty compared with first keratoplasty [2, 3]. Activation of nuclear factor kappa B (NF-kB) plays a key role in the pathogenesis of rejection of transplants of various organs and tissues, primarily due to production of proinflammatory cytokines (TNF, IL-1, etc.) [4]. In addition, transcription factor NF-kB, a regulator of gene expression, plays a central role in oxidative injury associated with graft rejection. NFkB is a rapid acting primary transcription factor which is involved in the regulation of cellular response to unfavorable environmental conditions, damage, inflammatory processes and immune stimulation. It is found in the cytoplasm of all cells; upon stimulation, NF-kB is translocated to the nucleus, and induces the expression of more than 400 genes involved in a plethora of functions, such as immune response, apoptosis and cell cycle. Therefore, NF-κB controls biologically important cell functions (particularly, but not only, innate and adaptive immunity processes in various disorders) either directly or indirectly [5-7]. It has been suggested that NF-kB inhibition may have a potential clinical value in the treatment of inflammatory and autoimmune disorders and transplant rejection [5, 8]. NF-kB activation plays a key role in the pathogenesis of graft rejection; the factor can be activated by stimuli like ischemic graft injury, presence of graft antigens, and influence of cytokines, e.g., TNF-α and IL-1 [9; 10]. Eventually, this activation triggers pathological processes (activation of endothelial cells and host T cells, maturation of dendritic cells (patients antigen-presenting immune cells)) leading to graft rejection. The purpose of the study was to assess the role of transcription factor NF-kB in lymphocyte subsets in the prediction of outcomes for patients undergoing repeat keratoplasty. Material and Methods Forty-six patients (46 eyes) who underwent repeat keratoplasty were included. Of these, 30 patients (group 1) developed an opacified graft, and 16 (group 2) obtained a clear graft. Patient age ranged from 32 to 88 years. Patients had a routine eye examination preoperatively. The control group comprised 20 somatically healthy volunteers who were comparable in age with patients. Imaging flow cytometry (ImageStream Mark II – AMNIS) and Amnis NF-kB Translocation Kits (ACS10000, Millipore, Sigma) were used to assess percentages of the cells with translocation of the NF-kB p65 subunit for for the following lymphocyte subsets: CD3+ (T-cells); CD3+CD4+ (Т-helpers); CD3+CD8+ (cytotoxic T-cells); CD3-CD19+ (В-cells); CD3- CD16+/CD56+ (NK cells); CD4+CD25+CD127high (activated Т-helpers – Tacts); CD4+CD25+CD127low (regulatory T-cells – Tregs); and CD4+CD161+CD3+ (Th17 cells). Flow cytometry was used to assess immunocytochemically energy metabolism in lymphocyte subsets by measuring the activities of succinate dehydrogenase (SDH), the mitochondrial Krebbs cycle and oxidative phosphorylation enzyme [11]. Anti-Hu NFκB (p50) Alexa Fluor® 488 (Amnis® NFκB Translocation Kit) was used to recognize NFκB p50, and 7-AAD, a fluorescence dye, was used for nucleus staining. CD19-PE, CD4-PE, CD8-PE, CD (16/56)-PE, CD127-PE, CD161-PE, CD3- ECD, CD4-PB, and CD25 PE-Cy7 Beckman Coulter monoclonal antibodies were used to distinguish a particular cell subset. Imaging flow cytometry was acquired on an Amnis ImageStream® Mark II imaging flow cytometer (Millipore Sigma), X40 magnification, with low flow rate using INSPIRE® software. Data analysis was performed using IDEAS® software (Amnis Corp., Seattle, WA). NF-κB nuclear translocation was quantitatively measured using the similarity feature which measures the similarity of p50 NF-kB fluorescence to 7-AAD nuclear staining on in-focus single cells. Statistical analyses were conducted using Statistica 13.0 (StatSoft, Tulsa, OK, USA) software. The Mann–Whitney test was used to assess significance of differences. Percentages of activated cells (with NF-kB translocation) are presented as median and 25th-75th percentiles. The level of significance p ≤ 0.05 was assumed. Results B-cells had the highest percentages of activated cells (with NF-kB translocation) among different lymphocyte subsets (Fig. 1) both for patients and controls. The percentage of activated cells (with NF-kB translocation) was significantly higher for B-cells than for NK cells, T helpers or cytotoxic T-cells (Fig. 1). In addition, NF-kB activity was higher in NK cells than in T-cells; and in T-helpers than in cytotoxic T-cells (Fig. 1).
The percentage of activated cells (with NF-kB translocation) was statistically significantly higher in all lymphocyte subsets (excepting B-cells) of patients who underwent repeat keratoplasty compared with controls (Table 1).
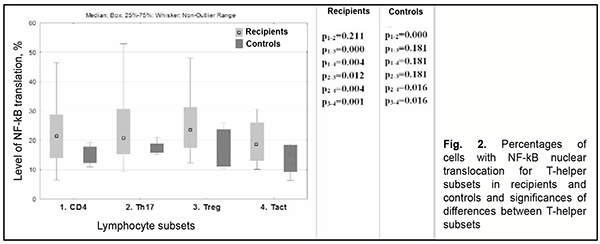
Among recipients, there was no significant difference in NFkB activity between Th17 cells and total T-helpers. In addition, compared to total T-helpers, NFkB activity was increased in Treg cells and decreased in Tact cells (Fig. 2). (Fig. 2).
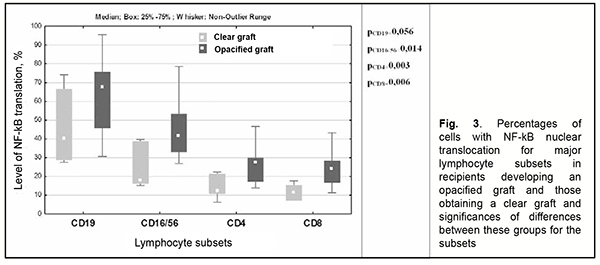
However, among controls, NF-kB activity in Th17 cells was significantly higher than in total T-helpers or activated T-helpers, but there was no significant difference in NF-kB activity between Th17 cells and regulatory T cells (Fig. 2). When we compared NF-kB nuclear translocation in patients developing an opacified graft following re-keratoplasty (group 1) to patients obtaining a clear graft following re-keratoplasty (group 2), we observed that lymphocytes of the former patients had 1.7-2.47 times higher levels of NF-kB nuclear translocation compared to the latter patients, with a particular amount of increase depending on the subset of lymphocytes, and the greatest increase was found for NK cells (Fig. 3).
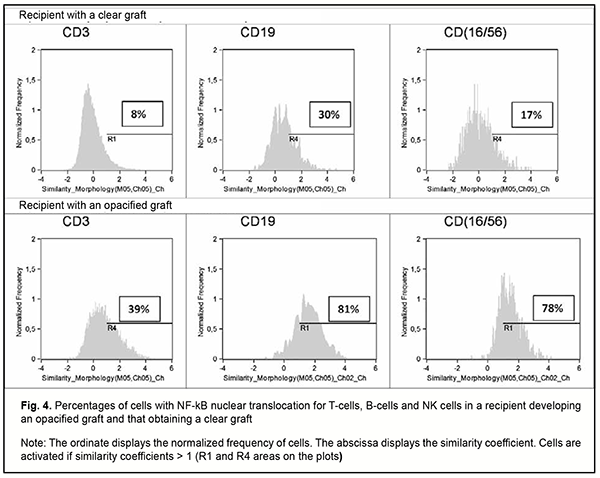
Figure 4 shows histograms of the frequency distributions of the major subsets of lymphocytes by NF-kB nuclear translocation (defined as the similarity score between NF-kB and 7-AAD staining) for patient D who obtained a clear graft, and patient G who developed an opacified graft, 12 months following re-keratoplasty (Fig. 4). In the patient who developed an opacified graft, there were significantly increased NF-kB nuclear translocation levels in T-cells, B-cells and NK cells.
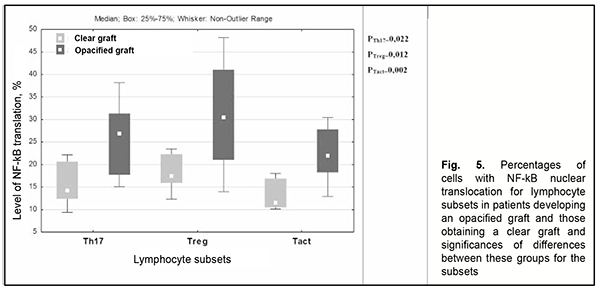
Analysis of minor subsets of lymphocytes demonstrated that the percentages of Th17 cells and activated T-helpers with NF-kB translocation were 1.7-times and 1.9-times, respectively, higher in patients who developed an opacified graft compared to those who obtained a clear graft following re-keratoplasty (Fig. 5).
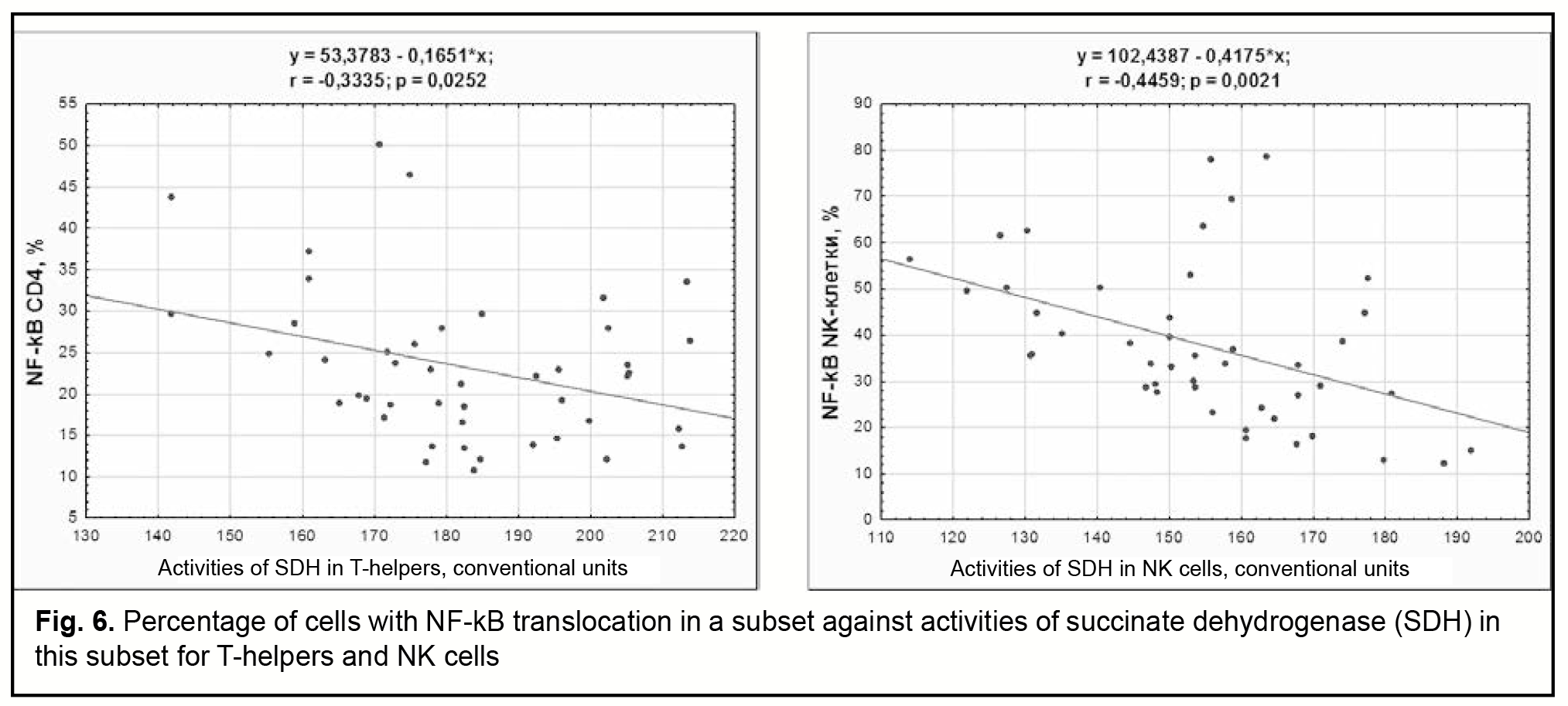
Our analysis of relations of the percentage of cells with NFkB translocation with the number of cells in the subset and the subset percentage (among total lymphocytes) found that B cells were the only subset of lymphocytes for which there was association between the percentage of cells with NFkB translocation and the subset percentage (among total lymphocytes). For this population, the percentage of activated cells increased with a decrease in the subset percentage (among total lymphocytes) (Pearson correlation, r=-0.31). In addition, the percentages of activated T-cells and T-helpers inversely correlated with activities of SDH in these subsets (Pearson correlations, r=-0.31 and r=-0.33, respectively, Fig. 6). Moreover, the percentage of NK cells with NF-kB nuclear translocation increased with a decrease in activities of SDH in these cells (Pearson correlation, r=-0.45, Fig. 6).
Discussion Corneal graft rejection, especially that following re-keratoplasty, is a complex pathophysiological process which has not been completely explored. Advances in experimental immunology in recent years have led to new insights into histoincompatibility response in primary and repeat keratoplasty. It is due to the immune privilege of the eye (particularly, special structural and functional relationships in the cornea and anterior segment of the eye) that a clear graft is obtained following keratoplasty in the absence of risk factors; this privilege is implemented by local and systemic mechanisms [2]. A compromised corneal immune privilege creates conditions for ‘switching on’ the mechanisms of transplant immunity, and is a major risk factor for the development of histoincompatibility response. An interaction between recipient cells, donor cells and the immune system triggers a sequence of reactions and mechanisms which lead to graft opacity. Accordingly, there is increasing importance of pre- and post-operative monitoring of the recipient’s immune system and identifying the most informative immunological characteristics for early detection of the potential development of graft rejection. Transcription factor NF-kB regulates innate and adaptive immunity responses and is a major mediator of inflammatory responses [5; 6]. NF-kB induces expression of pro-inflammatory genes, including those that encode cytokines and chemokines. Non-regulated activation of NF-kB contributes to pathogenic processes in different inflammatory disorders and plays a key role in the pathogenesis of various organs and tissues [4]. Introduction of imaging flow cytometry in recent years has enabled quantitative assessment of NF-kB activation in cell subsets in transplants, and it was demonstrated that the level of NF-kB translocation in T-cells can be used to monitor therapeutic immune suppression after transplant [12]. In the current study, the highest levels of NF-kB nuclear translocation were observed in B-cells and NK cells both for recipients and controls. Substantially increased levels of NF-kB nuclear translocation were found in the major subsets (T-cells and NK cells) and minor subsets (Th17, Treg, Tact) of patient lymphocytes compared to control lymphocytes. There were substantially increased levels of NF-κB nuclear translocation in all subsets of lymphocytes (excepting B-cells) of patients who developed an opacified graft compared to those who obtained a clear graft following re-keratoplasty, with the most apparent increase found for NK cells. Because an increase in NF-kB activity precedes graft rejection [9], we believe that the level of NF-kB translocation in NK cells may be used for monitoring patients following repeat keratoplasty. The importance of monitoring subsets of T helpers (like Th1 cells, Th17 cells, and regulatory T cells) for the diagnosis and prediction of the host response to the graft has been noted [13]. We have previously demonstrated [14] that the percentage of Th17 cells (from total CD4) in recipients with graft rejection was increased compared to patients with a clear graft both during corneal transplantation and throughout the follow-up, with no difference in the percentage of regulatory T cells (from total CD4) between the two groups of patients. However, we would like not only to be able to perform quantitative assessment of lymphocyte subsets, but also to have some criteria for assessing their functional activity. Activation of transcription factor NF-kB in cell subsets is a potential criterion of functional activity of these subsets. We found that the percentages of cells with NF-kB translocation among Th17 cells, regulatory T cells and activated T helpers in patients developing an opacified graft were significantly increased compared to patients with a clear graft following re-keratoplasty. Therefore, an opacified graft was observed in patients with an increased percentage and activation of Th17 cells and activation of regulatory T cells. Interestingly, that NF-kB activity did not correlate with the percentage of cells for T cells or NK cells and inversely correlated with the percentage of B cells. It is likely due to the fact that changes in the NF-kB activity of cells are more abrupt than changes in the percentage of these cells. Recent studies have assessed the relationships of the level of NF-kB translocation with the functional activity of the mitochondria and metabolic intensity [15, 16]. It has been reported that the transcription factors NF-kB control mitochondrial metabolism through the classical pathway of transcription activation, with the p65 subunit of NF-kB being able to penetrate mitochondria, bind to the mitochondrial DNA, and inhibit the expression of cytochrome oxidase (complex IV of oxidative phosphorylation), thus contributing to a switch from oxidative phosphorylation to glycolysis. We found that the level of NF-kB translocation inversely correlated with metabolic intensity assessed through the activity of succinate dehydrogenase in T cells and NK cells: the activity of succinate dehydrogenase decreased with an increase in the level of NF-kB translocation. Therefore, an increase in the level of NF-kB translocation in subsets of T cells (NK cells, Th17 cells, and regulatory T cells) is an early risk factor for corneal graft rejection.
References 1.Perez VL. Visualization of immune responses in the cornea. Cornea. 2017 Nov;36 Suppl 1:S5-S8. 2.Neroev VV, Balatskaya NV, Chentsova EV, Shamkhalova KhM. [Mechanisms of immune regulation and transplantation immunity in corneal transplants]. Medical Immunology (Russia)/Meditsinskaia Immunologiia. 2020; 22(1): 61-76. Russian. 3.Kitazava K, Wakimasu K, Kayukawa K, Yokota I, Inatomi T, Hieda O, et al. Moderately long-term safety and efficacy of repeat penetrating keratoplasty. Cornea. 2018 Oct;37(10):1255-9. 4.Kuncevich NV. [Role of nuclear factor NF-kB in graft rejection]. Vestnik transplantologii i iskusstvennykh organov. 2017;12;1:72-7. Russian. 5.Liu T, Zhang L, Joo D, Sun SC. NF-kB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. 6.Sun SC, Chang JH, Jin J. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 2013 Jun;34(6):282-9. 7.Yue Y, Stone S, Lin W. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen Res. 2018 Sep;13(9):1507-15. 8.Park MH, Hong JT. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016 Mar 29;5(2):15. 9.Troulfas G, Geller DA. NF‐κB in transplantation: friend or foe? Transpl Infect Dis. 2001 Dec;3(4):212-9. 10.Serasanambati M, Chilakapati SR. Function of Nuclear Factor Kappa B (NF-kB) in Human Diseases—A Review. South Indian J Biol Sci. 2016;2:368. 11.Patent RF, MPK G01 № 33/53, G01 № 33/50. [Method for measuring the mitochondrial activity of lymphocytes]. Petrichuk SV, Izmailova TD, Radygina TV. The applicant and patent holder is the National Medical Research Center for Children’s Health of the Russian Ministry of Health. № 2005141145/15; declared 28.12.2005; published 10.07.2007. Russian. 12.Sommerwerck U, Lindemann M, Kleibrink BE, Rabis T, Weinreich G, Kamler M, et al. NF-kB location in T-cells is promising to monitor immunosuppression after lung transplantation. Eur Resp J. Sep 2014; 44 (Suppl 58):3309. 13.Onishchenko NA, Bashkina LV, Nikolskaia AO, Artamonov SD. [Informative value of CD4 monitoring for the diagnosis and prognosis of host-graft response]. Vestnik transplantologii i iskusstvennykh organov. 2013; 15(4): 112-25. Russian 14.Komakh YA, Borzenok SA, Petrichuk SV, Samokhina IV. [Corneal graft rejection: clinical-and-anamnestic and immunological criteria for diagnosis]. Rossiiskii Immunologicheskii Zhurnal. 2017;11(20);4:714-716. Russian. 15.Albensi BC. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front Cell Dev Biol. 2019 Aug 7;7:154. 16.Angajala A, Lim S, Phillips JB, Kim J-H, Yates C, You Z, Tan M. Diverse roles of mitochondria in immune responses: novel insights into immune-metabolism. Front Immunol. 2018 Jul 12;9:1605.
Funding: This study did not receive any funding support.
|