J.ophthalmol.(Ukraine).2021;1:55-61.
|
http://doi.org/10.31288/oftalmolzh202115561 Received: 20 August 2020; Published on-line: 12 February 2021 Clinical picture of uveitis in the presence of ocular hypertension and improvement in disease course with dipeptide carnosine I. M. Mikheytseva, N. V. Bondarenko, S. G. Kolomiichuk, T. I. Siroshtanenko SI «The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine»; Odesa, Ukraine E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mykheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. Clinical picture of uveitis in the presence of ocular hypertension and improvement in disease course with dipeptide carnosine. J.ophthalmol.(Ukraine).2021;1:55-61. http://doi.org/10.31288/oftalmolzh202115561 Background: Our previous studies have demonstrated that raised IOP can contribute to an increase in the severity of inflammatory processes in the anterior segment. Given the involvement of free radical processes in the pathogenesis of uveitis, it is reasonable to use antioxidative agents for the treatment of the disease. The naturally occurring dipeptide carnosine (beta-alanyl-L-histidine) is a highly bioavailable low-molecular hydrophilic antioxidant with a direct effect on certain oxidant species and indirect effect on the system protecting the body from radicals; it contributes to membrane stabilization and can improve inflammatory processes. Purpose: To examine the features of the clinical course of experimental anterior uveitis developing in the presence of ophthalmic hypertension (OHT), and to improve the course with dipeptide carnosine. Material and Methods: Thirty-four rabbits were divided into three groups (group 1, 10 animals with induced allergic uveitis; group 2, 12 animals with OHT induced prior to experimental allergic uveitis; and group 3, 12 animals treated with carnosine for experimental allergic uveitis in the presence of OHT). Animals underwent biomicroscopy, ophthalmoscopy and tonometry. The number of white blood cells (WBC) in the aqueous humor of the anterior chamber was assessed microscopically, and total protein concentration was determined with the Lawry assay. Results: There were significant differences in the conjunctival and scleral injection (р=0.0001), pattern of keratic precipitates (р=0.0009), anterior chamber content (р=0.0034), pattern of posterior synechiae (р=0.0025), vitreous opacity (р=0.0338), and fundus pathology (р=0.0001) between groups of anterior uveitis-only and that with OHT. Even at four weeks of induced anterior uveitis, the number of WBC and the total protein in the aqueous humor of the anterior chamber were 14.3% (p > 0.05) and 27.5%, respectively, higher in in eyes of group 2 compared to eyes of group 1. The course of inflammatory process was significantly more severe in eyes with anterior uveitis in the presence of OHT than in eyes with uveitis-only, based on the comparison in major clinical signs in the anterior and posterior segments. Binocular instillation of carnosine solution in the conjunctival sac for 4 weeks resulted in a significant decrease in the severity of inflammatory process in the anterior and posterior segments in eyes of group 3, leading to an improved clinical picture and restoration of the blood-aqueous barrier and ciliary body transport, with a 45.8% decrease in the WBC and 31.6% decrease in total protein in the aqueous humor of the anterior chamber in treated eyes compared to untreated eyes with induced non-infectious anterior uveitis in the presence of OHT (р < 0.01). Conclusion: Integration of carnosine into the therapeutic regimen for ocular inflammation in the presence of raised IOP would substantially improve treatment outcomes and reduce the complication rate. Keywords: anterior uveitis, ocular hypertension, carnosine, rabbits
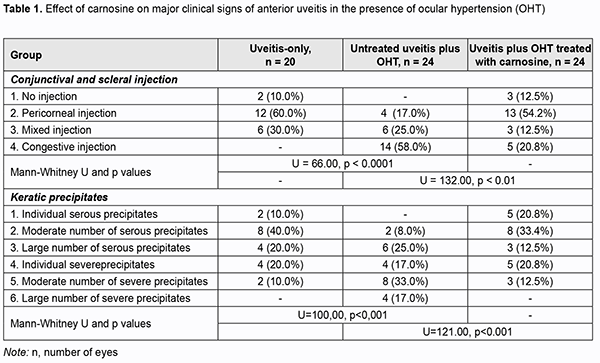
Introduction An increase in the prevalence of uveitis is still a problem in ophthalmology, and is caused by several factors including an increase in the prevalence in working-age individuals. The disease leads to low vision or blindness in 9% to 39% of patients [1]. Uveitis is idiopathic in most cases. Patients with uveitis tend to have various comorbidities. The disease can be caused by a natural body response to infection. An increased intraocular pressure (IOP) may be a major factor that complicates the course of anterior uveitis. In a study by Herbert and colleagues (2004) [2], the prevalence of raised IOP in eyes with uveitis was approximately 42%. In a study by Kopaienko (2010) [3], the prevalence of ocular hypertension (OHT) in eyes with chronic uveitis was approximately 40% (39/98 eyes) [3]. Free radical processes and lipid peroxidation are important in the pathogenesis of inflammatory diseases, whereas oxidative stress is a major pathogenetic factor in the pathogenesis of glaucoma process [4-7]. The results of our previous studies have revealed an important aspect of the pathogenetic effect of raised IOP on the severity of uveal inflammation through activation of oxidative and peroxidative processes in uveal structures, and confirmed the hyposesis that raised IOP is a factor contributing to the severity of inflammation in the anterior eye [8]. The metabolic abnormalities found in these studies indicated that oxidative stress is an aspect of the pathogenetic effect of ophthalmic hypertension (OHT) as a factor contributing to the severity of inflammation in the anterior eye. Given the metabolic features of anterior uveitis (particularly those of the anterior uveitis associated with glaucoma) and the involvement of free radical processes in the pathogenesis of these disorders, treatment of the latter should include antioxidative medications. Currently available medications for the treatment of uveitis (particularly those for the disease in the presence of OHT) are not sufficiently effective. While looking for novel effective and safe agents for the treatment of uveitis, we considered the naturally occurring dipeptide carnosine (beta-alanyl-L-histidine) [9-11]. The purpose of the study was to examine the features of the clinical course of experimental anterior uveitis developing in the presence of OHT, and to improve the course with dipeptide carnosine. Material and Methods Chinchilla rabbits, weighing 3.0 to 3.5 kg, maintained under normal vivarium conditions and fed and watered ad libitum, were used for all experiments. All animal experiments were performed in compliance with the General Ethical Principles of Animal Experiments (approved by Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986). Thirty-four rabbits were divided into 3 experimental groups (group 1, 10 animals with induced allergic uveitis; group 2, 12 animals with OHT induced prior to experimental allergic uveitis; group 3, 12 animals treated with carnosine for experimental allergic uveitis in the presence of OHT). Allergic uveitis was induced by introducing bovine serum albumin at a dose of 5 mg into the conjunctival cavity only after the animal was sensitized [12]. OHT was induced by a single 0.1-mL injection of 0.3% carbomer into the anterior chamber of the rabbit. Carnosine was introduced by instilling 5% solution into the conjunctival sac of each eye in animals of group 3, twice daily for the four weeks. General anesthesia was administered by intramuscular injection of ketamine hydrochloride (50 mg/kg), and topical anesthesia was performed by instillation of 0.5% proparacaine hydrochloride into the conjunctival sac one minute before injection. Animals underwent biomicroscopy, ophthalmoscopy and tonometry. We used a Maklakoff tonometer with a 7.5-g plunger load to perform IOP measurements after the eye was subjected to topical anesthesia by instillation of 0.5% proparacaine hydrochloride into the conjunctival sac. Mean IOP values were 13.5 ± 0.6 mmHg for the control group, 18.7 ± 1.2 mmHg for induced OHT, 15.3 ± 1.4 mmHg for induced anterior uveitis, and 23.4 ± 1.3 mmHg for induced OHT with uveitis. The number of white blood cells (WBC) in the aqueous humor of the anterior chamber was assessed microscopically, and total protein concentration was determined with the Lawry assay [13]. Non-parametric methods (SPSS 11 and Statistica 5.5 software) were used for statistical analysis. Results In the first phase of the experiment we examined the effect of raised IOP on the development of anterior uveitis in rabbits. Tables 1-3 present the features of the clinical course of anterior uveitis developed in the presence of OHT. There were significant differences in the conjunctival and scleral injection (р=0.0001), pattern of keratic precipitates (р=0.0009), anterior chamber content (р=0.0034), pattern of posterior synechiae (р=0.0025), vitreous opacity (р=0.0338), and fundus pathology (р=0.0001) between groups of anterior uveitis-only and that with OHT.
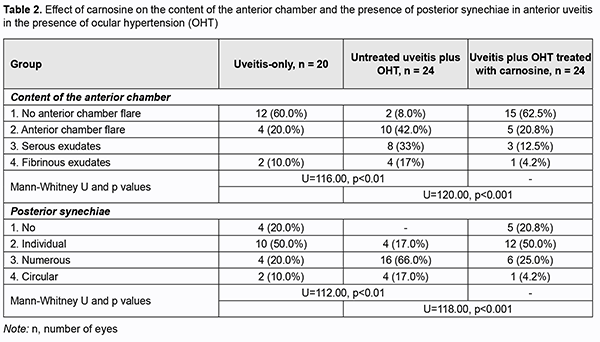
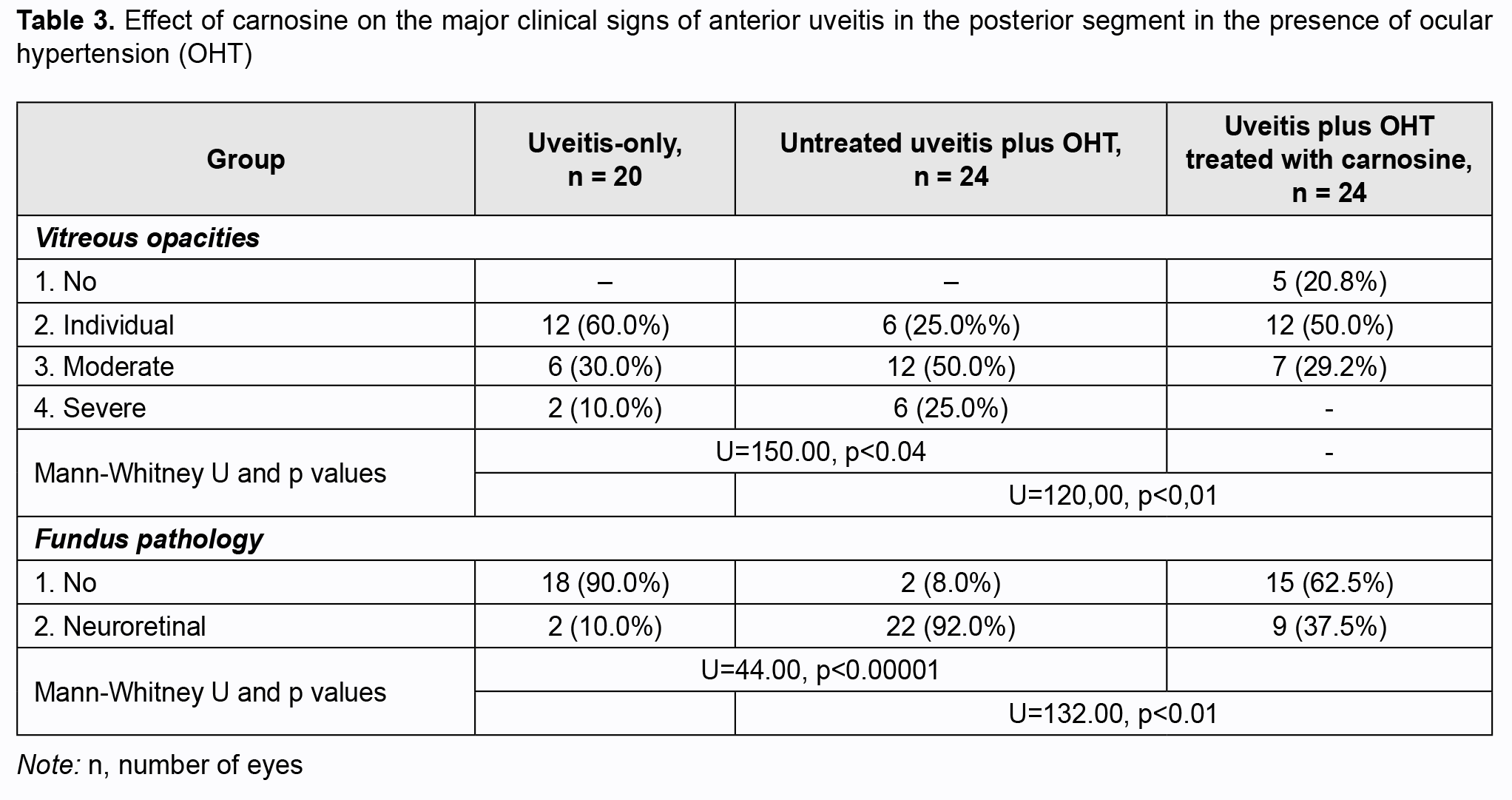
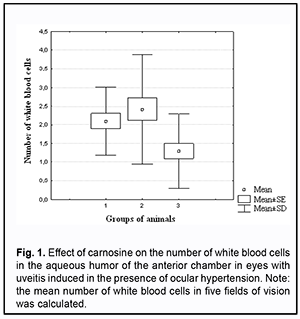
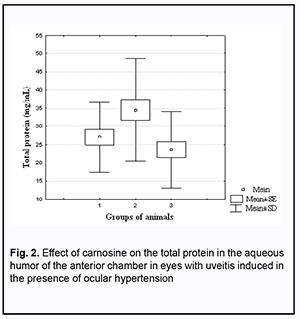
Of the eyes of group 1 (i.e., uveitis-only), 10% showed no pathologic changes, 60%, pericorneal injection, and 30%, mixed injection. Eyes of group 2 (uveitis with OHT) showed more apparent changes; of these eyes, 17% exhibited pericorneal injection, 20%, mixed injection, and 58%, congestive injection (Table 1). Keratic precipitates were more numerous and severe in eyes of group 2 (anterior uveitis with OHT) than in eyes of group 1 (anterior uveitis-only). Thus, a moderate number of severe keratic precipitates was seen in 10% of eyes of group 1 and 33% of eyes of group 2, whereas a large number of severe keratic precipitates was seen in 17% of eyes of group 2, and not seen in eyes of group 1. In addition, the percentage of eyes exhibiting profound changes in the anterior chamber was higher in group 2 (anterior uveitis with OHT) than in group 1 (anterior uveitis-only): no anterior chamber flare, 8% vs 60%; anterior chamber flare, 42% vs 20%; serous exudates, 33% vs 10%; fibrinous exudates, 17% vs 10%. Posterior synechiae were not seen in 20% of eyes of group 1, whereas, of the eyes of group 2, 66% showed multiple posterior synechiae, and 17%, circular posterior synechiae (vs 10% for group 1) (Table 2). The state of the anterior eye was also assessed with the use of laboratory characteristics (ocular vascular permeability as assessed by the number of white blood cells (WBC) and total protein in the aqueous humor of the anterior chamber) (Figs 1 and 2). It has been previously demonstrated that these indices are increased in eyes with inflammation [14, 15].
Of note that even at four weeks of induced anterior uveitis, the number of WBC in the aqueous humor of the anterior chamber was substantially increased, but these cells were absent in the aqueous humor of the anterior chamber in normal eyes and in eyes with induced OHT. The number of WBC in the aqueous humor of the anterior chamber was 14.4% higher in group 2 (anterior uveitis with OHT) than in group 1 (anterior uveitis-only) (р > 0.05). At four weeks of induced anterior uveitis, the total protein in the aqueous humor of the anterior chamber was 44-fold higher in group 1 (anterior uveitis-only) compared with control group eyes (0.62 ± 0.05 mg/mL). In addition, the total protein in the aqueous humor of the anterior chamber was 27.5% higher in group 2 (anterior uveitis with OHT) compared with group 1 (anterior uveitis-only) eyes. The presence of WBC as well as a significantly increased total protein in the aqueous humor of the anterior chamber in group 1, and, especially, in group 2, at 4 weeks, indicated an increased permeability of vascular system in the anterior eye (the iris and ciliary body) and progression of ocular inflammation. We also assessed the state of the posterior segment in rabbit eyes with induced non-infectious uveitis (Table 3). The percentage of eyes exhibiting individual, moderate and severe vitreous opacities in group 2 (anterior uveitis with OHT) was 25%, 50%, and 25%, respectively, vs 60%, 30%, and 10%, respectively, in group 1 (anterior uveitis-only). Of note that fundus pathology was absent in 90% of group 1 eyes, whereas 92% of group 2 eyes exhibited neuroretinal changes. Therefore, we experimentally demonstrated that anterior uveitis development was more apparent in rabbit eyes with raised IOP than in those with normal IOP. OHT contributed to the severity of inflammatory process in the anterior eye. Since carnosine has a high antioxidative and anti-inflammatory capacity [16-20], we experimentally assessed its effect on the clinical and laboratory signs of anterior uveitis developing in the presence of OHT. Tables 1 to 3 show the data on the effect of carnosine on the main clinical signs of anterior uveitis developing in the presence of ocular hypertension. Among the rabbit eyes treated with carnosine for induced anterior uveitis in the presence of OHT, the percentage of eyes exhibiting mixed injection (12.5%) was smaller than for group 2 (eyes not treated for induced anterior uveitis in the presence of OHT), 20.8% of eyes showed congestive injection, and 12.5%, no injection. In addition, among the rabbit eyes treated with dipeptide carnosine for induced anterior uveitis in the presence of OHT (group 3), the incidence of eyes with a great number of keratic precipitates was lower, whereas that with individual keratic precipitates was lower than among the untreated eyes (group 2) (Table 1). There were significant differences in conjunctival and scleral injection (р < 0.05) and pattern of keratic precipitates (р < 0.05) between groups of untreated and treated eyes with induced anterior uveitis in the presence of OHT. Treatment with carnosine contributed to a significantly decreased incidence of eyes with serous and fibrinous exudates and anterior chamber flare compared to untreated eyes with induced anterior uveitis in the presence of OHT, with 62.5% of eyes showing no anterior chamber flare versus 8.0% for untreated eyes (Table 2). In addition, treatment with carnosine of eyes with induced anterior uveitis in the presence of OHT contributed to a significantly decreased incidence of eyes with circular or multiple posterior synechiae, increased incidence of eyes with individual posterior synechiae, and with some eyes (20.8%) showing no posterior synechiae, compared to untreated eyes (Table 2). There were significant differences in the anterior chamber content (р < 0.05) and pattern of posterior synechiae (р < 0.03) between groups of untreated and treated eyes with induced anterior uveitis in the presence of OHT. Moreover, treatment with carnosine contributed to improved laboratory characteristics of the aqueous humor of the anterior chamber (Figs 1 and 2), with a 45.8% decrease in WBC count (р < 0.01) and 31.6% decrease in total proten (р < 0.01) in the aqueous humor compared to untreated rabbit eyes with induced anterior uveitis in the presence of OHT. Table 3 presents the data on the effect of carnosine on the state of the posterior segment in eyes with anterior uveitis developing in the presence of OHT. Thus, there was no incidence of severe vitreous opacity among the eyes treated with dipeptide carnosine, compared to 25% for untreated eyes with induced anterior uveitis in the presence of OHT. In addition, the percentage of eyes with moderate opacities was decreased (29.2% vs 50%), whereas that of eyes with individual opacities was increased (50% vs 25%), compared to untreated eyes, and there were eyes without opacity (20.8%) among treated, but not untreated eyes. Fundus pathology was absent in 62.5% of group 3 eyes, whereas the rest (37.5%) of group 3 eyes exhibited neuroretinal changes. Discussion In the current study, OHT caused an increase in the severity of inflammation in rabbit eyes with induced anterior uveitis, with significant increases in incidences of eyes with conjunctival and scleral injection, precipitates (a great number of severe keratic precipitates was seen in 17% of eyes of group 2, and not seen in eyes of group 1), fibrinous exudates in the anterior chamber (17% vs 10%) and numerous posterior synechiae (66% vs 20%). In addition to changes in the anterior segment tissues, rabbit eyes with both raised IOP and induced non-infectious anterior uveitis showed substantial changes in the posterior segment (particularly, vitreous opacity and changes in the neural retina) at 4 weeks of the experiment. Moreover, rabbit eyes with both raised IOP and induced non-infectious anterior uveitis showed an increase in WBC number and a 27.5% increase in the total protein in the aqueous humor of the anterior chamber compared with anterior uveitis-only eyes, which indicated an eye tissue trophic disorder and blood-ocular barrier dysfunction. When considering the mechanisms of uveal damage in ocular inflammatory processes, especially in conjunction with other pathogenic factors like OHT, it should be noted that increased free radical processes and increased lipid peroxidation may play a key role in the pathogenesis of infectious and allergic uveitis [19-21]. Particularly, phagocytes in the focus of inflammation may play a role in the presence of activated xanthine oxidase, NADPH-oxidase and myeloperoxidase, and with hyperproduction of superoxide and hydroxyl radicals, which, if produced excessively, have not only an immunoregulatory effect, but also a cytotoxic effect [22, 23]. Previous animal studies [14,15] on the effect of instillation of dexamethasone and superoxide dismutase have demonstrated the efficacy of antioxidants for reduction in inflammatory process and breakdown of the blood-aqueous barrier in experimental anterior uveitis. Treatment of rabbits with antioxidative agents, lipoflavon and acetylcysteine, for OHT induced in the presence of allergic uveitis was found to prevent abnormal activity of antioxidant enzymes in the anterior chamber angle tissue and increased lipid peroxidation in the aqueous humor [24]. The present animal study demonstrated that antioxidative treatment with carnosine contributed to a substantial decrease in the severity of pathologic changes in tissues of the anterior and posterior segments in eyes with induced non-infectious anterior uveitis in the presence of OHT. Binocular instillation of carnosine solution in the conjunctival sac for 4 weeks resulted in a significant decrease in the severity of inflammatory process in the anterior and posterior segments, leading to an improved clinical picture and restoration of the blood-aqueous barrier and ciliary body transport, with a 45.8% decrease in the WBC and 31.6% decrease in total protein in the aqueous humor of the anterior chamber in treated eyes compared to untreated eyes with induced non-infectious anterior uveitis in the presence of OHT (р < 0.01). Therefore, we believe that integration of carnosine into the therapeutic regimen for ocular inflammation in the presence of raised IOP would substantially improve treatment outcomes and reduce the complication rate.
References 1.Grechanyĭ MP, Chentsova OB, Kil'diushevskiĭ AV. [Ethiology, pathogenesis and prospects for treating autoimmune eye diseases]. Vestn Oftalmol. Sep-Oct 2002;118(5):47-51. Russian. 2.Herbert HM, Viswanathan A, Jackson H, Lightman SL. Risk factors for elevated intraocular pressure in uveitis. J Glaucoma. 2004;13:96–9. 3.Kopaienko AI. [Ocular hypertension in patients with endogenous anterior uveitis: risk factors and treatment]. Tavricheskiĭ mediko-biologicheskiĭ vestnik. 2010; 13(1):113-5. Russian. 4.Kurysheva NI, Vinetskaia MI, Erichev VP, Demchuk ML, Kuryshev SI. [Contribution of free-radical reactions of chamber humor to the development of primary open-angle glaucoma].Vestn Oftalmol. 1996 Sep-Oct;112(4):3-5. Russian. 5.Alekseev VN, Martynova EB, Sadkov VI. [Role of peroxidation in the pathogenesis of primary open-angle glaucoma]. Oftalmol Zh. 2000;1:12-7. Russian. 6.Ko M, Peng P, Ma M. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005 Aug 1;39(3):365-73. 7.Ielskiĭ VN, Mykheitseva IM. [Dysregulatory aspects of the glaucomatous process (review of the literature and own investigations]. Zhurnal NAMN Ukrainy. 2011;17(3):235-44. Russian. 8.Mikheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. Oxidation and peroxidation in the uvea of the rabbit eyes with experimental uveitis and ocular hypertension. J Ophthalmol (Ukraine). 2019;4:57-63. 9.Boldyrev AA, Aldini G, Derave W. Physiology and Pathophysiology of Carnosine. Physiol Rev. 2013 Oct;93(4):1803-45. 10.Babizhayev МА, Kasus-Jacobi A. State of the Art Clinical Efficacy and Safety Evaluation of N-acetylcarnosine Dipeptide Ophthalmic Prodrug. Principles for the Delivery, Self-Bioactivation, Molecular Targets and Interaction With a Highly Evolved Histidyl-Hydrazide Structure in the Treatment and Therapeutic Management of a Group of Sight-Threatening Eye Diseases. Curr Clin Pharmacol. 2009;4(1):4-37. 11.Volkov OA. [Biological role of carnosine ant its use in ophthalmology (minisurvey of literature)]. Biomeditsinskaia khimiia. 2005;51(5):481-4. Russian. 12.Information Bulletin No. 19 issued 10.10.2019, based on Pat. of Ukraine №137,107; MPK (2019.01) А61К 9/00. [Method for inducing non-infectious uveitis in the presence of ocular hypertension]. Authors: Mykheitseva IM, Kolomiichuk SG, Bondarenko NV, Siroshtanenko TI. Owner: State Institution Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine. 13.Larson E, Howlet B, Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243-8. 14.Chesnokova NB, Neroev VV, Beznos OV, Beyshenova GA, Panova IG, Tatikolov AS. [Effects of dexamethasone and superoxide dismutase instillations on clinical course of uveitis and local biochemical processes (experimental study)]. Vestn Oftalmol. 2015 May-Jun;131(3):71-5. Russian. 15.Pavliuchenko KP, Kravtsova NB. [Effect of thiotriazolin and acetylcysteine in the intensity of inflammatory process in experimental uveitis]. Oftalmol Zh. 2006;6:46-9. Russian. 16.Babizhayev MA, Yermakova VN, Sakina NL. N-alpha-acetylcarnosine is a prodrug of L-carnosine in ophthalmic application as antioxidant. Clin Chim Acta. 1996 Oct 15;254(1):1-21. 17.Min J, Senut MC, Rajanikant K, et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008 Oct;86(13):2984-91. 18.Oganesian AA, Minasian AG, Seiranian VM, Akopian VE. [Efficacy of N-acetylcarnosine in diabetic retinopathy]. Med nauka Armenii, NAN RA. 2009;2:57-67. Russian. 19.Iarygina EG, Prokopieva VD, Bokhan NA. [Oxidative stress and its improvement with]. Uspekhi sovremennogo estestvoznaniia. 2015;4:106-13. Russian. 20.Babizhayev M. A. Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin. 2016 Dec;19:49–68. 21.Savko VV, Khelifi Amani, Parkhomenko TV. [Effect of allergic uveitis on peroxidation characteristics of lipids of the aqueous humor of the anterior chamber in experimental glaucoma]. Oftalmol Zh. 2010;6:52-4. Russian. 22.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB Journal. 1995;9:200–9. 23.Karbyshev MS, Abdullaiev ShP. Editor, Shestopalov AV. [Biochemistry of Oxidative Stress: A Guidance Manual]. Pirogov Russian National Research Medical University: Moscow. 2018; Russian. 24.Savko VV, Khelifi Amani. [Lipid peroxidation and state of enzymatic antioxidant system in experimental hypertension in animals with allerfic uveitis treated with lipoflavon and acetylcysteine]. Oftalmol Zh. 2011;4:61-6. Russian. The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|