J.ophthalmol.(Ukraine).2021;1:38-45.
|
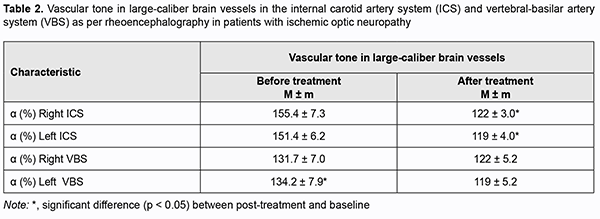
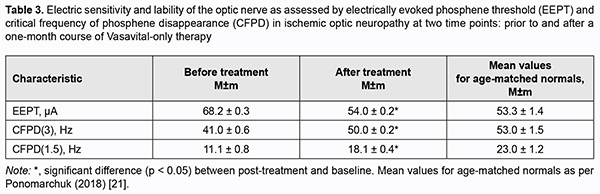
http://doi.org/10.31288/oftalmolzh202113845 Received: 02 November 2020; Published on-line: 12 February 2021 Clinical experience of the use of Vasavital® in dry age-related macular degeneration and ischemic optic neuropathy V. S. Ponomarchuk, N. I. Khramenko, N. V. Konovalova The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: kolomiets.wa@gmail.com TO CITE THIS ARTICLE: Ponomarchuk VS, Konovalova NV, Khramenko NI. Clinical experience of the use of Vasavital® in dry age-related macular degeneration and ischemic optic neuropathy. J.ophthalmol.(Ukraine).2021;1:38-45. http://doi.org/10.31288/oftalmolzh202113845 Background: The chronic ischemic process caused by impaired hemodynamics in the eye and brain is a component of the pathogenesis of ischemic optic neuropathy and age-related macular degeneration, and requires prompt and adequate treatment. Purpose: To examine the effect of a one-month course of Vasavital on the function of the visual system and regional hemodynamics in patients with dry age-related macular degeneration and those with ischemic optic neuropathy. Material and Methods: Thirty-two patients with dry age-related macular degeneration (AMD) (mean age, 66 ± 1.5 years) and 22 patients with chronic ischemic optic neuropathy (mean age, 57.0 ± 1.4 years) underwent examination and treatment at the Department of Uveitis and Laboratory for Functional Examination of the Visual System of the Filatov institute. They received a one-month course of Vasavital-only therapy at a dose of one capsule twice a day as an outpatient treatment. Patients reported their complaints and underwent routine eye examination, studies of regional hemodynamics in the eye and brain (by ophthalmic rheography and rheoencephalography with Reocom, the computerized rheography apparatus), and electrophysiological studies of electrically evoked phosphene threshold (EEPT) and critical frequency of phosphene disappearance (CFPD) at two time points: prior to and after a one-month course of Vasavital-only therapy. Results: A one-month course of Vasavital-only therapy resulted in 24% and 9.6%-25% increases in ocular pulse blood filling in patients with chronic ischemic optic neuropathy and patients with age-related macular degeneration with various baseline levels of visual acuity, respectively. In addition, vascular tone in large-and small-caliber ocular vessels decreased significantly, by 12-17%, in patients with chronic ischemic optic neuropathy, and by 10-20%, in patients with age-related macular degeneration, and vascular tone in large-caliber brain vessels (in the vertebral-basilar system) decreased by 21.6% in patients with ischemic optic neuropathy. A course of Vasavital-only therapy resulted in improved optic nerve function in patients with ischemic optic neuropathy, with a 20.5% improvement in electrically evoked phosphene threshold (EEPT), and 22-63% improvement in critical frequency of phosphene disappearance (CFPD); this indicated an improved function of the peripheral as well as central retina. Keywords: Vasavital, dry age-related macular degeneration, ischemic optic neuropathy
The basic Principles of the Predictive, Preventive and Personalized Medicine as the Healthcare Model of the near future, as well as the Strategy for the Development of Medical Science in Russia for the Period until 2025, require that researches in healthcare (particularly, but not only, eye care) shall focus on the areas associated with the genetic, cellular, nano- and information technologies [1]. The emergence of fundamentally novel technological solutions in the field of gene therapy and gene diagnostics, the creation of priority for and development of genetic technologies create serious prerequisites for the beginning of a new Fusion era in ophthalmology in the near future. Before we overview current genetic technologies in ophthalmology and make the analysis of global trends in these technologies, we consider current normative legal support and maintenance in the area of developing genetic technologies. The Federal Research Program for Genetic Technologies Development until 2027 was approved in 2019 [2]. A Program’s key objective is to implement a comprehensive solution to the task of the accelerated development of genetic technologies, including genetic editing. The meeting of the Working Group on Legal Regulation in the Area of Genetic Technologies, Including Genetic Editing and Bioethics on October 16, 2020, adopted a decision on the establishment of the Center for Law and Bioethics Related to Genomic Research and Technologies, the principal aim of which is to provide legal support for the accelerated development and application of genetic technologies. The Center for Precision Genetic Editing and Genetic Technologies in Biomedicine has been established within the framework of the program and the national project named “Science”. Gene therapy. Gene delivery systems The gene delivery systems can be divided into two classes, viral and non-viral; either has its advantages and disadvantages. Viral gene delivery systems Recombinant-deficient viral vectors (viruses that are no longer able to replicate) are commonly used for the delivery of genetic material into cells. The types of vectors available for the delivery include adenoviruses, adeno-associated viruses, lentiviruses and herpes simplex viruses. Adenoviruses have a rather high capacity for gene transfer (their vectors can carry long fragments of DNA, more than 20,000 base pairs, or 20 kb), a high level of transduction (DNA transfer to target cells), and their viral DNA does not integrate into the host cell DNA (the genetic material remains in the cytoplasm and is not delivered into the nucleus, which is both an advantage and a disadvantage, because transgene expression is transient); in addition, they are highly immunogenic (not safe) [3, 4]. Recombinant adeno-associated viruses (AAV) are attractive vectors for several reasons: they are non-pathogenic, have a low immunogenicity, display broad tropism to most cells and tissues, high transduction efficacy, integrate into the host DNA at the particular site, and can trigger long-term transgene expression. One limitation of recombinant AAV is that their genome-packaging capacity is not ≤ ~ 5 kb. Experimental studies on the use of AAV for induced neovascularization models of retinal and optic nerve disorders (age-related macular degeneration (AMD), pigmented retinitis, Leber’s amaurosis and Stargardt disease) have been widely conducted in recent years [5-19]. Retinal, corneal, and trabecular meshwork transductions have been found to be effective for the various AAV variants and various routes of AAV delivery. Boye and colleagues [20] used a mixture of viscoelastic (Healon) and AAV to place sub-inner limiting membrane (ILM) injections in the primate retina, and demonstrated uniform and extensive transduction of retinal ganglion cells (RGCs) in the areas beneath the subILM bleb. Simpson and colleagues [21] suggested that AAV-PHP.eB, a novel AAV9-based mutant capsid, crosses the intra-retinal blood-retinal barrier (IR-BRB), efficiently transduces horizontal cells located adjacent to IR-BRB, but has very limited ability to further penetrate retina and reach photoreceptors. Lee and colleagues [22] evaluated the transduction efficiencies of four different AAV serotypes, AAV2, 5, 8, and 9, in streptozotocin (STZ)-induced diabetic mouse retinas. AAV2 and AAV9 displayed the most efficient transduction. Particularly, AAV2 was transduced into various retinal cells, including Müller cells, microglia, retinal ganglion cells (RGCs), bipolar cells, horizontal cells, and amacrine cells, whereas AAV9 was effectively transduced only into RGC and horizontal cells [22]. In a mouse study by Wang and colleagues [23], a synthetically developed AAV transduced following a single intracameral injection efficiently the trabecular meshwork, corneal stroma, and endothelial cells. In a mouse study by Lee and colleagues [24], laser photocoagulation induced transduction of retinal cells by intravitreally administered AAV. Lentiviruses (LV) belong to the family of retroviruses, have a rather small genome-packaging capacity (≤8 kb), can trigger long-term transgene expression, and induce minimal host immune response. However, they randomly integrate into the genome and can cause insertional mutagenesis by disrupting other genes, leading to severe adverse events. In ophthalmological studies, lentiviruses have been less frequently used for genetic material delivery than AAV. They have been used for vitreoretinal disorders, AMD, diabetic retinopathy, choroidal melanoma, retinoblastoma, neovascularization, and corneal dystrophy [11, 25-29]. In an interesting rat study by Bai and colleagues [30], LV mediated toll-like receptor 2 (TLR2) small interference RNA (SiRNA) could effectively down regulate the TLR2 expression via RNA interference and prolong the survival of corneal grafts, although not necessarily able to prevent the rejection. Therefore, currently, fundamental studies on the use of AAV and LV for the delivery of genetic material into the eye are widely conducted. Particularly, they found that: (1) retinal, corneal, and trabecular meshwork transductions are effective for the various AAV variants and various routes of AAV delivery; (2) different AAV serotypes differ in transduced cells and tissues of the eye; (3) the efficacy of genetic therapy mostly depends on the route of delivery. As at August, 2019, according to online Wiley library records (http://www.abedia.com/wiley/indications.php), the total number of clinical studies on gene therapy drugs was 3000. Of these, 95% were phase 1/2 studies, and 5% were phase 2/3 studies. In addition, as at August, 2019, postmarketing studies were being conducted only for 5 gene therapy drugs. Only 9 gene therapy drugs have been approved for use by the Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA). Luxturna (Spark Therapeutics, Philadelphia, PA), an AAV-based vector carrying a normal copy of the RPE65 gene to the RPE cells that are lacking a normally functioning RPE65 gene, was approved for the treatment of biallelic RPE65mutation–associated retinal dystrophy (Leber congenital amaurosis) by the FDA in 2017, and by the Committee for Medicinal Products for Human Use (CHMP) of the EMA in 2018. Luxturna is injected subretinally once per eye, for patients who have confirmed RPE65 mutations and enough viable cells in their retina [31, 32]. The first commercial gene therapy with Luxturna was performed at the Children's Hospital Los Angeles in 2018. Efficacy and safety studies of AAV-mediated delivery of the genetic material to the eye have been widely conducted in recent years. GenVec (Gaithersburg, MD) assessed the safety of a single intravitreous injection of an E1-, partial E3-, E4-deleted adenoviral vector expressing human pigment epithelium-derived factor (AdPEDF.11) for advanced neovascular AMD in a phase I clinical trial [33]. There were no serious adverse events related to AdPEDF.11, but signs of mild, transient intraocular inflammation occurred in 25% of patients. Avalanche Biotechnologies (Menlo Park, CA) in collaboration with the Australian Lions Eye Institute investigated the safety profile and effectiveness of subretinal injections of rAAV sFLT-1 recombinant AAV (rAAV) soluble fms-like tyrosine kinase-1 (sFLT-1) for neovascular AMD in Phase 1 and 2a human clinical trials [34, 35]. A subretinal injection of a low-dose (1×1010 vector genomes (vg)) or high-dose (1×1011 vg) sFLT-1 was found to be safe, and administration of anti-VEGF gene therapy for neovascular AMD was found to be promising. CD59 complement factor is a membrane-bound inhibitor of the membrane attack complex (MAC), an immune protein that mediates cell lysis by the formation of plasma membrane pores. Hemera Biosciences (Waltham, MA) carried out a phase I gene therapy trial to evaluate the safety of MAC inhibition via intravitreal delivery of an adeno-associated virus vector (AAV2) that expresses the soluble form of membrane-independent CD59 (sCD59), AAVCAGsCD59 (HMR59), in participants with dry AMD [36]. The trial found HMR-59 to be safe and effective, and, of the patients with at least 6 months of therapy, 18% did not require anti-VEGF treatment within this period [37]. Oxford BioMedica (UK) Ltd carried out a phase I trial in patients with advanced NVAMD, the Gene Transfer of Endostatin/angiostatin for Macular Degeneration Trial (GEM Study), to test the safety and bioactivity of subretinal injection of a lentiviral Equine Infectious Anemia Virus (EIAV) vector expressing the angiogenesis inhibitors endostatin and angiostatin (RetinoStat®) [27]. The study findings demonstrated that lentiviral EIAV vectors provide a safe platform with robust and sustained transgene expression for ocular gene therapy. Adverum Biotechnologies (Menlo Park, CA) started a phase I trial to study the safety profile of intravitreal ADVM-022, a novel recombinant AAV, in 2018. ADVM-022 utilizes the AAV2.7m8 capsid, which carries a strong, ubiquitous expression cassette encoding a codon-optimized cDNA of the aflibercept protein. Patients with AMD and active CNV had an intravitreal injection of 6E11 vg/eye or 2 6E11 vg/eye as per the study protocol. The study is still ongoing and the final results have not been presented as yet. A phase 1/2 study evaluated the safety and tolerability of RGX-314 (RegenexBio) in patients with wet AMD. RGX-314 is an AAV8 vector encoding for a soluble anti-VEGF Fab protein, which binds to RPE cells to produce a therapeutic anti-VEGF protein. The gene encodes for an anti-VEGF fragment of a monoclonal antibody. The safety of different doses of RGX-314 (3E9 GC, 1E10 GC, 6E10 GC, 1.6E11 GC, and 2.5E11 GC) was evaluated in a phase 1 trial. Stabilization of the process was observed in 73% of the cases within 6 months, and in 50% of the cases within 18 months, and patients did not require anti-VEGF treatment within these periods. A long-term follow-up study of RGX-314 (RGX-314 LTFU) was initiated in December, 2019. Non-viral gene delivery systems These include plasmid DNA that is either delivered directly (termed “naked DNA”) or complexed with carriers (e.g., metal-based, polymer-based or lipid-based nanoparticles). Non-viral gene delivery systems may offer a less invasive, low immunogenic and inflammatory response than viral systems and can package rather large plasmids. What is more, and important to consider, they are more attractive for manufacturers because their manufacturing cost is lower and they are more easily standardized. The major disadvantage is low efficiency of transfection of the genetic material into a cell. Naked DNA is the simplest gene delivery system. In this case, they use a gene which is joined by a relatively low number of nucleotide sequences. Although there have been relatively few reports on studies with naked DNA, they found the reporter plasmid DNA to be efficiently transduced and transfected into retinal cells [38, 39]. Metal-based, polymer-based and lipid-based nanoparticles are more common and successful gene delivery systems compared to naked DNA. Metal-based (cerium oxide and yttrium oxide) nanoparticles are non-toxic for ocular tissues and provide antioxidant protection in retinal disorders [40-44]. Polymer-based nanoparticles have recently attracted the attention of researchers because they can be rather easily manufactured and have controllable characteristics. Particular attention is paid to the CK30-PEG compacted DNA nanoparticles that have been successfully tested in the eye. These particles have been demonstrated to be non-immunogenic in various tissues. In addition, in several studies, they have been used for gene transfer in models of retinitis pigmentosa and diabetic retinopathy, and were supposed to be promising for these disorders [45, 46]. Lipid-based delivery systems (liposomes, DNA-lipid complexes, and etc.) are currently one of the most commonly used types of nanoparticle carriers. Gao and colleagues [47] constructed the orongoxygen induced retinopathy (OIR) mouse (C57BL/6J) model and evaluated targeting VEGF siRNA transfection by new polymeric liposomes to inhibit retinal neovascularization. They found this approach to produce a durable therapeutic effect, with a significantly reduced area of retinal neovascularization [47]. A disadvantage of lipid-based delivery systems is the lack of cell specificity which may result in non-target effects. Wang and colleagues [48] demonstrated that cell-specific promoters enable lipid-based nanoparticles to deliver genes to specific cells of the retina in vivo. A novel method of targeted delivery of the genetic material has been recently developed, which enables transfection of rather large gene constructs. The technique utilizes polymer-covered gold nanorods and near-infrared laser irradiation, with gold nanoparticles having a maximum absorption spectrum in this region. These nanorods are heated with application of a laser pulse, and, when nanoparticles are in contact with the cell membrane, the nanorods enable site-specific transfection of large gene constructs due to increased permeability of the cell membrane without non-target effects [49].
Conclusion Therefore, the results of ophthalmological clinical studies demonstrate that applications of gene therapy drugs delivered by viral (mainly, AAV) and non-viral systems are safe and effective, and that gene therapy seems promising for the treatment of various eye disorders. We, however, need to bear in mind that (a) AAV can show immunogenicity (especially, associated with repeat injections), (b) LLV can cause insertional mutagenesis and carcinogenesis, and (c) liposome-based delivery systems do not always allow for cell-specific transport mechanisms, which can produce non-target effects. Today, the major objectives in the area of systems for gene therapy delivery to the eye are targeted delivery of the genetic material and transduction/transfection of large therapeutic genes.
References 1.Supuran CT. Agents for the prevention and treatment of age-related macular degeneration and macular edema: a literature and patent review. Expert OpinTher Pat. 2019;24:1–7. 2.Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25(10 Suppl):S172–S181. 3.Gorusupudi A, Nelson K, Bernstein PS. The Age-Related Eye Disease 2 Study: Micronutrients in the treatment of macular degeneration. Adv Nutr. 2017;8:40–53. 4.Khoo HE, Ng HS, Yap WS, et al. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants (Basel). 2019;8(4).pii:E85. 5.Bourne RR, Jonas JB, Bron AM, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102:575–585. 6.Pawlowska E, Szczepanska J, Koskela A, et al. Dietary Polyphenols in Age-Related Macular Degeneration: Protection against Oxidative Stress and Beyond. Oxid Med Cell Longev. 2019;2019:9682318. 7.Roberts JE, Dennison J. The Photobiology of Lutein and Zeaxanthin in the Eye. J Ophthalmol. 2015;2015:687173. 8.Martínez-Solís I, Acero N, Bosch-Morell F, et al. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Med. 2019 Jul 2. 9.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017;7:CD000254. 10.Murillo AG, Hu S, Fernandez ML. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants (Basel). 2019;8(9).pii:E390. 11.Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients. 2017;9(2).pii:E120. 12.Coscas G, Cunha-vaz J, Loewenstein A, Soubrane G. Macular edema: a practical approach. Basel, Switzerland: Karger; 2010:1-9. Developments in Ophthalmology; vol 47. 13.Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017;139:123–37. 14.Lawler T, Liu Y, Christensen K, et al. Dietary Antioxidants, Macular Pigment, and Glaucomatous Neurodegeneration: A Review of the Evidence.Nutrients. 2019;11(5).pii:E1002. 15.Miller JW. Developing Therapies for Age-related Macular Degeneration: The Art and Science of Problem-solving: The 2018 Charles L. Schepens, MD, Lecture. Ophthalmol Retina. 2019;3(10):900–9. 16.Chew EY, Clemons TE, Sangiovanni JP, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–9. 17.Denisova IP. [On the role of ischemic and involutionary factors in the pathogenesis of ocular degenerative disorders]. In: [Fedorov memorial lectures- 2014]. Moscow;2014. p.128-204. Russian. 18.Frolov MA, Alkam KM. [Manifestation of ocular ischemic syndrome in patients with atherosclerotic carotid artery stenosis]. In: [Proceedings of the East-West-2013 Conference]. Ufa;2013. p.279. 19.Moisseiev E, Loewenstein A. Drug Delivery to the Posterior Segment of the Eye. Dev Ophthalmol. 2017;58:87–101. 20.Occhiutto ML, Freitas FR, Maranhao RC, Costa VP. Breakdown of the Blood-Ocular Barrier as a Strategy for the Systemic Use of Nanosystems. Pharmaceutics. 2012;4(2):252–5. 21.Ponomarchuk VS. [Diagnostics using electrical phosphenes in ophthalmology]. Odesa: Astroprint; 2018. Russian. 22.Montes P, Ruiz-Sánchez E, Rojas C, RojasP. Ginkgo biloba extract 761: a review of basic studies and potential clinical use in psychiatric disorders. CNS Neurol Disord DrugTargets. 2015;14:132-49. 23.Yin B, Xu Y, Wei R, LuoB. Ginkgo biloba on focal cerebral ischemia: a systematic review and meta-analysis. Am J Chin Med. 2014; 42(4):769-83. 24.Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high-tension glaucoma. Mol Vis. 2012; 18: 390-402. 25.Spadiene A, Savickiene N, Jurgeviciene N, Zalinkevicius R, Norkus A, Ostrauskas R, Skesters A, Silova A, Rodovicius H, Francaite-Daugeliene M. Effect of ginkgo extract on eye microcirculation in patients with diabetes. Cent Eur J Med. 2013; 8: 736-41. 26.Park JW, Kwon HJ, Chung WS, Kim CY, Seong GJ. Short-term effects of Ginkgo biloba extract von peripapillary retinal blood flow in normal-tension glaucoma. Korean J Ophthalmol. 2011; 25: 323-8. 27.Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999 Jun;15(3):233-40. 28.Khramenko NI, Konovalova NV, Guzun OV. Regional and central hemodynamics in ischemic optic neuropathy. J Ophthalmol (Ukraine). 2018;3:3-9. This papper was supported by "Ukrayinsʹka farmatsevtychna kompaniya", LLC (Ukrainian pharmaceutical company, LLC)
|