J.ophthalmol.(Ukraine).2021;1:24-31.
|
http://doi.org/10.31288/oftalmolzh202112431 Received: 30 June 2020; Published on-line: 12 February 2021 Cytological features of the bulbar conjunctiva in patients with type 2 diabetes mellitus T. M. Zhmud,1 G. I. Drozhzhyna,2 A. V. Demchuk 3 1 Pirogov Vinnytsia National Medical University; Vinnytsia (Ukraine) 2 The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) 3 Podilsky Regional Center of Oncology of the Vinnytsia Regional Council"; Vinnytsia (Ukraine) E-mail: gtatyana@email.ua TO CITE THIS ARTICLE: Zhmud TM, Drozhzhyna GI, Demchuk AV. Cytological features of the bulbar conjunctiva in patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2021;1:24-31. http://doi.org/10.31288/oftalmolzh202112431 Background: Pathologic changes in the ocular surface in diabetes mellitus (DM) may result from various causes such as metabolic abnormalities (hyperglycemia and dyslipidemia, leading to changes in tear composition), perilimbal vascular microangiopathy (leading to impaired blood supply to the cornea), lacrimal gland lesions, and various abnormalities in innervation. Purpose: To determine the cytological features of the bulbar conjunctival epithelium in patients with T2DM. Material and Methods: We analyzed clinical and impressive cytology data of 34 patients (18 (52.9%) men and 16 (47.1%) women; age, 50 to 79 years) having clinical signs of dry eye disease (DED). Patients were divided into two equal groups of 17 patients each, group 1 (diabetes duration of less than 5 years) and group 2 (diabetes duration of more than 5 years). They underwent Schirmer 1 test, tear break-up time (TBUT) test, and impression cytology of the bulbar conjunctiva. The cytologic changes of the bulbar conjunctiva were graded according to Nelson’s grading system. Results: Comparison of clinical and cytolological characteristics of study patients suggests that, severity, duration and course pattern of diabetes mellitus affect the development of compensatory and adaptive mechanisms in the bulbar conjunctival epithelium, these mechanisms being reflected by the formation of the surface epithelium of increased density due to squamous metaplasia and keratinization. Conclusion: Our impression cytology study of the bulbar conjunctival epithelium found that most patients (94.1%) with T2DM had squamous metaplasia grade (Nelson) of 2 or 3, and 80% of those with squamous metaplasia grade (Nelson) of 3 had T2DM duration of more than 5 years. In addition, squamous metaplasia of grade 3 was fourfold more common among patients with T2DM duration of more than 5 years than among those with T2DM duration of less than 5 years. Keywords: impression cytology, bulbar conjunctiva, type 2 diabetes mellitus
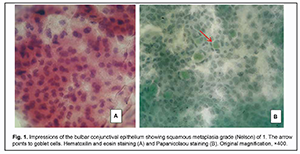
Introduction Type 2 diabetes mellitus (T2DM) has been defined as “a chronic disease that occurs when the pancreas does not produce enough insulin, or when the body cannot effectively use the insulin it produces”. Diabetes mellitus leads to complications such as neuropathy, retinopathy, nephropathy, cardiovascular disorders, and other metabolic disorders, in which hyperglycemia plays a major role [1]. In addition, it has been found to be a major factor in dry eye disease (DED). A correlation was found between the glycated hemoglobin (HBA1c) values and the presence of dry eye syndrome. The higher the HBA1c values, the higher the rate of dry eye syndrome [2]. Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film stability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles [3]. Pathologic changes in the ocular surface in DM may result from various causes such as metabolic abnormalities (hyperglycemia and dyslipidemia, leading to changes in tear composition), perilimbal vascular microangiopathy (leading to impaired blood supply to the cornea), lacrimal gland lesions, and various abnormalities in innervation [4]. Diabetic patients exhibit lesions of the afferent sensitive nerves, and efferent autonomic and motor nerves [5]. Dry eye disease has traditionally been classified into two categories: aqueous deficient (dry eye with reduced tear production) and evaporative (dry eye with increased evaporation of the tear film). However, these categories are not mutually exclusive, and patients with diabetes-induced dry eye may have a combination of these mechanisms of DED (i.e., mixed DED) [6-8]. Chronic hyperglycemia, diabetic periphery neuropathy, decreased insulin levels, microvasculopathy, and systemic hyperosmotic disturbances are risk factors for diabetes-associated DED, which leads to abnormal tear production and meibomian gland dysfunction. Several proteins have been identifies as contributors to diabetes mellitus-associated DED [9]. We have previously found that DED severity was correlated with T2DM duration. Mild dry eye symptoms were found approximately 1.5-fold more frequently in patients with diabetes duration longer than 5 years than in those with diabetes duration shorter than 5 years [10]. Numerous studies on the pathogenesis of have found that meibomian gland dysfunction is a major factor in dry eye disease [10]. A combination of the two mechanisms of DED was common among patients with T2DM, and 61.3% of these patients were found to have palpebral demodicosis [11]. The prevalence of DED has been reported to vary from 7% to as high as 98.8% [4]. In a study by Figueroa-Ortiza and colleagues [12], DED in diabetic patients was more frequently determined on the basis of objective signs (fluorescein and lissamine green staining test) than on the basis of subjective signs. The Beaver Dam Eye Study reported that approximately 20% of dry eyes occurred in individuals with T2DM aged 43 to 86 years. In a study by Hom and de Land [13], a total of 52.9% of those with either diabetes or borderline diabetes had self-reported clinically relevant dry eyes. A confocal miscroscopy study by Grigorieva and colleagues [4] found corneal nerve fiber lesions (i.e., reduced fiber number, thin and tortuous nerve fibers) in diabetic patients even without signs of diabetic retinopathy. The bulbar conjunctiva is known to be covered with the multilayer squamous epithelium with goblet cells secreting mucin. In humans, mucous and ocular surfaces are covered and protected by glycated protein which is secreted by goblet cells and exogenous glands. Ocular metabolic and neurotrophic changes in diabetes result in corneal epithelial and conjunctival lesions, leading to reduced goblet cell number and low mucin production, which eventually causes tear film instability [6, 14]. Conjunctival impression cytology is a method used to verify morphological changes in the ocular surface, including those in DED. The technique refers to the application of cellulose acetate Millipore filter to the ocular surface to remove the superficial layers of the ocular surface epithelium. The cells thus removed can be subjected to histological, immunohistological, or molecular analysis [15, 16]. Impression cytology is low invasive, easy and fast to use, and enables repeat sample taking. Impression cytology represents a non- or minimally invasive biopsy of the ocular surface epithelium with no side effects or contraindications. It has demonstrated to be a useful diagnostic aid for a wide variety of processes involving the ocular surface. In addition, and mainly during the last decade, its use as a research tool has experienced an enormous growth and has greatly contributed to the understanding of ocular surface pathology [17]. Thus, impression cytology found changes in epithelial and goblet cells of patients with DED. Thus, in a study by Nelson and Wright [18], compared with normal eyes, eyes with DED demonstrated a 17% greater goblet cell loss on the interpalpebral bulbar compared with the inferior palpebral ocular surface. Another study, by Petrayevsky and Trishkin [19], demonstrated signs of squamous conjunctival metaplasia in patients with mild and severe DED. In addition, impression cytology demonstrated the presence and severity of squamous metaplasia of the palpebral conjunctival epithelium and ocular surface epithelium in contact lens wearers [20, 21], and a marked reduction in bulbar conjunctival goblet cells in the Down’s syndrome group compared with the group of healthy controls [22]. Dry eye patients with hypothyroidism exhibited degenerative changes in epithelial and goblet cells in an impression cytology study of the bulbar conjunctiva, and these changes were more apparent in patients with manifest disease than in those with subclinical disease [23]. Conjunctival impression cytology is an essential diagnostic tool in ocular surface disorders. The cell density and characteristics of the conjunctival surface may differ according to localization, and the changes in ocular surface disorders are first observed in bulbar than in palpebral conjunctiva [24]. The technique is interesting due to its capacity to detect changes in the conjunctival mucosa of the globe and the palpebral mucosa for the diagnosis of disorders associated with ocular surface disease. Citirik and colleagues [24] found such ocular surface changes as squamous metaplasia of the bulbar conjunctiva in patients with diabetic retinopathy, but did not divide these patients into those with type 1 and type 2 diabetes mellitus, although these types differ in the pathogenesis, age of patients and disease progression. The purpose of this study was to determine the cytological features of the bulbar conjunctival epithelium in patients with T2DM. Material and Methods We analyzed clinical and impressive cytology data of 34 patients (18 (52.9%) men and 16 (47.1%) women; age, 50 to 79 years) with T2DM having clinical signs of DED. Patients were divided into two equal groups of 17 patients each, group 1 (diabetes duration of less than 5 years) and group 2 (diabetes duration of more than 5 years). Of the 34 patients, 22 had a blood glucose level of < 8 mmol/L, and 12 had a blood glucose level of > 8 mmol/L. Patients underwent Schirmer 1 test and tear break-up time (TBUT) test. Impression cytology was performed by applying a strip of cellulose acetate Millipore filter to the nasal quadrant of the bulbar conjunctiva. The filter strip was gently pressed against the conjunctiva and removed with a peeling motion. Samples were immediately fixed in 95% ethyl alcohol, stained with hematoxylin and eosin or with the Papanicolaou stain, mounted on glass slides and cover-slipped with balsam [25]. The slides were examined by light microscopy (ECLIPSE-Е 200; Nikon, Tokyo, Japan) at magnification of x100 and x400 to assess epithelial cell shape, arrangement, nuclei, cytoplasm, and nucleus-to-cytoplasm ratio. In addition, we assessed whether the intercellular spaces were preserved, and whether and how many goblet cells and inflammatory cells were present in the field of vision. The cytologic changes were graded according to Nelson’s grading system (1983) [7, 17, 19, 25] as follows: Grade 0: Small and round epithelial cells with a nucleo-cytoplasmic ratio of 1:2, large nuclei and eosinophilic cytoplasm; abundant, plump, oval goblet cells with well-preserved intercellular spaces; Grade 1: Slightly larger and more polygonal epithelial cells with nucleo-cytoplasmic ratio 1:3 and eosinophilic cytoplasm. The nuclei are smaller. The goblet cells are decreased in number; however, they still maintain their plump and oval shape. The intercellular spaces are somewhat widened at some sites; Grade 2: Larger and polygonal, occasionally multi nucleated epithelial cells with variable staining cytoplasm and a nucleo-cytoplasmic ratio 1:4-1:5. Smaller and less intensely PAS-positive goblet cells with poorly defined cellular borders and markedly decreased in number. The intercellular spaces are widened and there is loss of intercellular bonds at some sites; Grade 3: Large polygonal epithelial cells with basophilic cytoplasm. Small, pyknotic and in many cells completely absent nuclei. There is loss of intercellular bonds and keratinization. Goblet cells absent. Grade 0 and 1 were regarded as normal whereas grade 2 and 3 as abnormal cytology of the bulbar conjunctiva. Irregular cell shape and nucleus size and positive cytoplasmic staining of epithelial cells, reduced number of absence of goblet cells, and widened intercellular spaces are signs of conjunctival squamous metaplasia. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine, and relevant laws of Ukraine. Results Impression cytology found severe pathological changes in bulbar conjunctival samples from all but two patients with T2DM. These two (5.8%) had a mild disease course, diabetes duration of less than 5 years, and a blood glucose level of < 8 mmol/L. Impression cytology revealed grade 1 Nelson changes in the bulbar conjunctival samples from these two patients, and showed sheets of round cells with reduced nuclei, a nucleo-cytoplasmic ratio of 1:3, and slightly widened intercellular spaces. In addition, there were about five plump goblet cells in the field of vision and small sites of polygonal epithelial cells with oxyphilic cytoplasm (Fig. 1). For the two patients, the mean Schirmer test score was 7.50 ± 0.20 mm/min, and mean TBUT value, 11.5 ± 0.10 s.
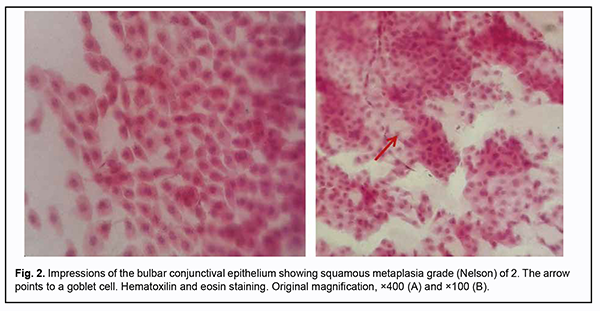
The bulbar conjunctival samples from 22 patients (64.7%) showed grade 2 Nelson changes in the epithelium, with sheets of polygonal and round cells with small nuclei and a nucleo-cytoplasmic ratio of 1:5, presence of multinuclear cells, and loss of intercellular bonds. In addition, these samples showed zero to two small goblet cells in the field of vision (Fig. 2). Of the 22 patients, 18 (81.8%) had a moderate diabetes and a blood glucose level of < 8 mmol/L. In addition, of these 18 patients, 10 (55.5%) had diabetes duration of more than 5 years. In patients with bulbar conjunctival samples showing grade 2 Nelson changes, the mean Schirmer test score was 7.0 ± 0.50 mm/min, and mean TBUT value, 4.5 ± 0.40 s.
Grade 3 Nelson changes were found in the bulbar conjunctival samples from 10 patients (29.4%). Of these 10 patients, 2 (20%) showed a severe diabetes, and 8 (80%), a moderate diabetes. In addition, 8 patients (80%) had diabetes duration of more than 5 years, and 9 (90%), a blood glucose level of > 8 mmol/L. Cytologically, the samples showed clear squamous metaplasia which was characterized by the presence of polygonal, elongated cells with small, pyknotic nuclei; binucleated cells; keratinization; high nucleo-cytoplasmic ratio; dystrophy (karyolysis, karyorrhexis, and vacuolized cytoplasm); loss of intercellular bonds; and absence of goblet cells (Fig. 3). In patients with bulbar conjunctival samples showing grade 3 Nelson changes, the mean Schirmer test score was 4.5 ± 0.50 mm/min, and mean TBUT value, 6.0 ± 0.50 s.
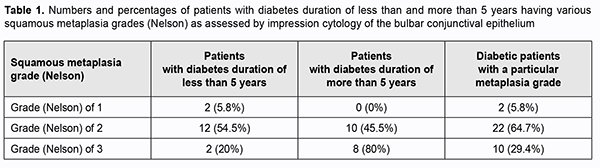
Table 1 shows the numbers and percentages of patients with T2DM duration of less than and more than 5 years among each squamous metaplasia grade (Nelson). Therefore, most patients (94.1%) had squamous metaplasia grade (Nelson) of 2 or 3, and squamous metaplasia of grade 3 was fourfold more common among patients with T2DM duration of more than 5 years than among patients with T2DM duration of less than 5 years.
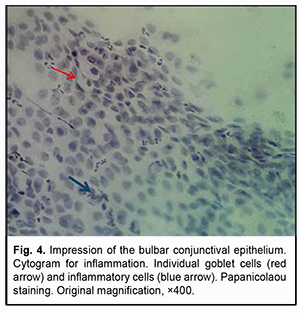
In addition, our cytological study revealed signs of conjunctival inflammation in samples from 4 patients (11.8%). These samples contained inflammatory infiltrations cells; of these cells, the most common were neutrophils (markers of acute inflammation), followed by degenerated epithelial cells and individual goblet cells having impoverished cytoplasm (Fig. 4).
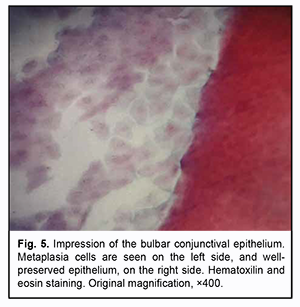
Almost all samples showed not only the above changes, but also sites of well-preserved epithelium at the impressions from deep layers of the bulbar conjunctiva. These sites had a potential for restoration of normal surface tissue structure (Fig. 5).
Comparison of clinical and cytolological characteristics of study patients suggests that, severity and duration of diabetes mellitus affect the development of compensatory and adaptive mechanisms in the bulbar conjunctival epithelium, these mechanisms being reflected by the formation of the surface epithelium of increased density due to squamous metaplasia and keratinization. In this case, metaplasia is positive due to its protective features, because in diabetes-associated DED, the conditions are created in which normal functioning of conventional mucosal cells becomes impossible, and the change of these cells to those non-conventional but more adapted to the pathological state is required. Discussion In a conjunctival impression cytology study, when compared with the healthy control group, diabetics showed more frequent and pronounced signs of conjunctival metaplasia [25]. Even though the “basic” unstimulated tear flow is normal in diabetics, the decrease in reflex tearing could be sufficient to induce chronic damage of the conjunctival surface, resulting in conjunctival metaplasia [25]. The trophic function of the tear film could be disturbed in diabetics, leading to chronic trophic damage of the conjunctival surface, neurotrophic keratopathy [25]. The ocular surface changes found in diabetics could at least partially be the result of a primary surface disease or of metabolic alterations of the conjunctival epithelial cells independent of tear film abnormalities. Petrayevsky and Trishkin [19] examined the cell content of the conjunctiva in patients with various severity of DED and demonstrated mild goblet cell loss in about half of these cases. These cytological findings give evidence on the presence of signs of conjunctival squamous metaplasia in patients with DED. Conjunctival squamous metaplasia has been reported in various DED-associated ocular surface disorders like burns, trachoma, and Sjögren syndrome [27]. In addition, some studied reported on the difference in the conjunctival flora between diabetics and non-diabetics. The development of conjunctivitis in patients with diabetes mellitus may be caused by pathological changes in the conjunctiva which have been found in a study by Citirik and colleagues [24]. Goebbels [26] observed ocular surface changes including squamous metaplasia of the bulbar conjunctiva in patients with diabetic retinopathy. Compared to patients with non-proliferative diabetic retinopathy (NPDR), those with proliferative diabetic retinopathy (PDR) exhibited higher impression cytology grading scores, but the difference was not significant [24]. Squamous metaplasia could at least partially be the result of a primary surface disease or of metabolic alterations of the conjunctival epithelial cells independent of tear film abnormalities [26]. Moreover, conjunctival hypoxia might also have played a role here. Significant associations have been identified between diabetic retinopathy (DR) and DED. In a hospital-based study, 17.1% of DED in patients with DM was found to have mild nonproliferative diabetic retinopathy (NPDR), 17.1% had moderate NPDR, 11.1% had severe nonproliferative diabetic retinopathy (NPDR), and 25.1% had proliferative diabetic retinopathy (PDR) [28]. Han and colleagues [1, 2] reported on the correlation between the progression and duration of diabetes mellitus. Although the mechanism of the development of DED in diabetes mellitus has not been completely elucidated, it is certainly is associated with metabolic abnormalities (hyperglycemia and dyslipidemia), various abnormalities in innervation and microangiopathy. Metabolic abnormalities developing in diabetes mellitus result in the transformation of the conjunctival epithelium, with squamous metaplasia, keratinization, and a loss of capacity to produce goblet cells as much as that leading to absence of these cells. This is evident by a decreased tear production and changes in the tear content pattern, consequently leading to the development of DED [6]. In the current study, we examined changes in the bulbar conjunctival epithelium in patients with T2DM, and found that the magnitude of changes depended on diabetes duration and blood glucose level. Conclusion Our impression cytology study of the bulbar conjunctival epithelium found that most patients (94.1%) with T2DM had squamous metaplasia grade (Nelson) of 2 or 3, and 80% of those with squamous metaplasia grade (Nelson) of 3 had T2DM duration of more than 5 years. In addition, squamous metaplasia of grade 3 was fourfold more common among patients with T2DM duration of more than 5 years than among those with T2DM duration of less than 5 years.
References 1.Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019; 14: 53–63. 2.Seifart U, Strempel I. [The dry eye and diabetes mellitus]. Ophthalmologe. 1994 Apr;91(2):235-9. German. 3.Winebrake JP, Drinkwater OJ, Brissette AR, et al. The TFOS Dry Eye Workshop II: Key Updates. Ophthalmic Pearls. CORNEA. 2017. 4.Grigorieva NN, Peich ME, Shadrichev FE. [Trehalose efficacy in dry eye syndrome therapy in diabetic patients]. Oftalmologicheskiie vedomosti. 2016;9(2):19–24. 5.Bezditko PA, Zavoloka OV, Lysenko MG. [Corneal changes in patients with diabetic retinopathy]. Oftalmol Zh. 2009;3:12-4. Ukrainian. 6.Swamynathan SK, Wells A. Conjunctival goblet cells: Ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul Surf. 2020 Jan;18(1):19-26. 7.Doughty MJ. Goblet cells of the normal human bulbar conjunctiva and their assessment by impression cytology sampling. Ocul Surf. 2012 Jul;10(3):149-69. 8.McKown RL, Wanf N, Raab RW, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009 May;88(5):848-58. 9.Zhang X, Zhao L, Deng S, et al. Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol. 2016;2016:8201053. 10.Zhmud ТМ, Malachkova NV, Andrushkova OO, et al. Meibomian gland dysfunction and dry eye disease symptoms in patients with type 2 diabetes mellitus. Reports of Morphology. 2019; 25(4):51-5. 11.Zhmud TM, Drozhzhina GI. Meibomian gland dysfunction accompanied by palpebral demodicosis in patients with type 2 diabetes mellitus. Journal of Ophthalmology. 2019; 6(491):23-8. 12.Figueroa-Ortiz LC, Jiménez Rodríguez E, García-Ben A, García-Campos J. Study of tear function and the conjunctival surface in diabetic patients. Arch Soc Esp Oftalmol. 2011 Apr;86(4):107-12. 13.Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77(11):554–558. 14.Masoud RM, Maryam R, Mohammad AA. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. 2008. – № 10 (2008). 15.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res. 2002;21:555–76. 16.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(4390):4391–8. 17.Calonge M, Diebold Y, Saez V, et al. Impression cytology of the ocular surface: a review. Exp Eye Res. 2004;78(3):457-72. 18.Nelson JDб Wright JC. Conjunctival Goblet Cell Densities in Ocular Surface Disease. Arch Ophthalmol. 1984;102(7):1049-1051. 19.Petrayevsky AV, Trishkin KS. [Clinical and cytological diagnostics of dry eye syndrome]. Vestnik VGU. 2012;4(44):52-4. Russian. 20.Egorova GB, Fedorov AA, Mitichkina TS, Shamsudinova AR. [Impression cytology changes in the conjunctival epithelial layer in patients with contact lens intolerance]. RMZh. Klinicheskaia oftalmologiia. 2014;1:17-9. Russian. 21.Egorova GB, Fedorov AA, Mitichkina TS. [Potential of impression cytology in diagnosis and evaluation of efficacy of pharmacological correction of dry eye syndrome associated with contact lens wearing]. Vestn Oftalmol. 2012 Jan-Feb;128(1):34-6. 22.Filippello M, Cascone G, Zagami A, Scimone G. Impression cytology in Down syndrome. Br J Ophthalmol. 1997;81(8):683–5. 23.Pavlovskyi MI, Vit VV, Drozhzhyna GI. [Citological features of the conjunctival epithelium in dry eye syndrome in patients with subclinical and manifest hypothyroidism]. Oftalmologiia. Vostochnaia Evropa. 2018;8(3):361-8. Russian. 24.Citirik M, Berker N, Haksever H, et al. Conjunctival impression cytology in nonproliferative and proliferative diabetic retinopathy. Int J Ophthalmol. 2014;7(2): 321–5. doi: 10.3980/j.issn.2222-3959.2014.02.23. 25.Singh R, Joseph A, Umapathy T, et al. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89:1655-9. 26.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000 Jan; 84(1): 19–21. 27.Gillan W. D. H. Conjunctival impression cytology: a review. S Afr Optom. 2008;67(3):136–41. 28.Nepp J, Abela C, Polzer I, et al. Is There a Correlation Between the Severity of Diabetic Retinopathy and Keratoconjunctivitis Sicca? Cornea. 2000 Jul;19(4):487-91.
The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|