J.ophthalmol.(Ukraine).2021;1:17-23.
|
http://doi.org/10.31288/oftalmolzh202111723 Received: 21 August 2020; Published on-line: 12 February 2021 Diagnosing meridional amblyopia in astigmats on the basis of assessment of asymmetries in visual acuity and refraction as vector quantities V. A. Kolomiyets, O. V. Kachan, N. V. Kolomiyets SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: kolomiets.wa@gmail.com TO CITE THIS ARTICLE: Kolomiyets VA, Kachan OV, Kolomiyets NV. Diagnosing meridional amblyopia in astigmats on the basis of assessment of asymmetries in visual acuity and refraction as vector quantities. J.ophthalmol.(Ukraine).2021;1:17-23. http://doi.org/10.31288/oftalmolzh202111723 Background: Asymmetry in refraction may cause meridional amblyopia and impairments in the mechanisms underlying binocular vision in patients with astigmatism. There have been contradictory reports on the features and incidence of meridional amblyopia in patients with astigmatism. These contradictions have been attributed to the fact that the studies vary in methodologies and criteria used for visual acuity assessment. Purpose: To improve the algorithm for diagnosing meridional amblyopia in patients with hyperopic astigmatism on the basis of assessment of asymmetries in meridional separable visual acuity and refraction as vector quantities. Material and Methods: Ninety three patients aged 6 to 12 years, with both refractive amblyopia and compound with-the-rule hyperopic astigmatism were included in the study, and underwent examination. The sphercal component of refraction ranged from 0.5D to 1.75D, and the astigmatic component of refraction, from 0.75D to 2.0D. Best-corrected visual acuity was assessed using letters and Landolt rings of Shevalev Chart and digits generated by a Hoya chart projector. Visual acuity characteristics were determined for the amblyopic eye, fellow eye and binocularly. Meridional separable visual acuity was measured with Landolt rings with the help of specially developed software. Optotypes were presented on the computer screen at a 5-m distance. Threshold meridional separable visual acuity was measured in the meridians corresponding to principal astigmatism axes under gradual change (with an increment of ±7.0 arc second) in the angular size of the optotype. Tests were presented monocularly and binocularly on a 15-inch 1600×1200-resolution display. Results: Graphic comparison of the results of visual acuity measurements using optotypes of different shapes in amblyopes with similar type of astigmatism demonstrated that, in the groups of patients with the same letter visual acuity, Landolt visual acuities can be higher, lower or equal to letter visual acuities. Of the study patients, 42.5% were found to have no meridional amblyopia, and 57.5%, to have meridional amblyopia, as assessed using Landolt rings. Particularly, 35% and 22.5% of the study patients had visual acuity in the horizontal meridian better and worse, respectively, than in the vertical meridian. Conclusion: Meridional separable visual acuities in patients with both amblyopia and similar type of hyperopic astigmatism are vector quantities and may vary in orthogonal retinal meridians not only in the magnitude, but also in the sign. Meridional visual acuity studies will allow a diagnosis of meridional amblyopia to be clarified not only based on the presence of asymmetry in visual acuity in orthogonal retinal meridians, but also based on the direction of asymmetry in visual acuity with respect to the principal astigmatic refraction meridians. Keywords: astigmatism, visual acuity, individual variations, meridional visual acuity, meridional amblyopia
Introduction Refractive abnormalities are a major cause of impaired function and development of the visual system. Patients with astigmatism are characterized by the most complex impairments in the mechanisms underlying binocular vision and visual perception. In astigmatism, the optical system of the eye forms images varying in size and sharpness across different retinal meridians, which results in a substantial impairment in the shape of object images. These factors cause the development of meridional amblyopia (MA). MA manifests itself as selective alterations in visual acuity (VA), with substantial differences in the ability to resolve contours and details of different orientations under conditions of best-corrected ametropia [1-4]. It is noteworthy that monocular meridional asymmetries in visual acuity cause serious impairments in the mechanisms underlying binocular vision [2, 3, 5-8]. Equal visual acuity in orthogonal retinal meridians in both eyes is required for reliable performance of the mechanisms. It is due to the fact that binocularly activated neurons represent the material basis for binocular vision mechanisms [4, 9]. Binocular neurons have and important feature, synergy: simultaneous stimulation of corresponding fields in both eyes produces a powerful pulse discharge, whereas monocular stimulation either produces a weak response of binocular cells or does not activate these cells. In meridional amblyopia, the above requirement is not met, which results in impaired function of the binocular system [4, 8-10]. Assessment of visual system resolution in astigmats will enable to (1) improve our understanding of the mechanisms of sensory system adaptation to between-eye asymmetry in refraction, (2) evaluate the preservation of various visual information processing channels and quality of optical correction of astigmatism, and (3) improve the strategy for diagnosis and management of meridional amblyopia [3, 5]. This article addresses the issue of how to clarify an algorithm for diagnosing amblyopia in astigmats on the basis of assessment of asymmetries in meridional visual acuity and refraction as vector quantities. Study rationale There have been contradictory reports on meridional VA in patients with astigmatism. Some studies demonstrated that meridional amblyopia in children can be found associated with hyperopic astigmatism [10-12], whereas others reported that children with hyperopic astigmatism showed no meridional amblyopia, but did show reduced acuity for both grating orientations [5, 13]. These contradictions have been attributed to the fact that the studies vary in methodologies and criteria used for visual acuity assessment [7, 14, 15]. These factors cause differences not only in meridional visual acuity, but also in results of any other visual acuity measurement methods [15, 16]. Variations in visual acuity with measurement methods can be explained on based on the current understanding of visual information processing mechanisms [17]. Classical visual acuity measurement algorithm involves presenting optotypes of various sizes and determining the threshold (i.e., the smallest) identifiable optotype size. The ability to identify a visual stimulus depends on its size and distance to it. The quantitative parameter unifying these two factors is the the visual angle of the stimulus [1, 17]. Various test images, optotypes (letters, digits, silhouette images, grating tests, Landolt rings, etc.) are used to measure visual acuity [16, 18, 19]. In order to standardize the results of the tests of various configurations, optotypes should have equal angular sizes, and designed so the width of the strokes and the gaps are one fifth of the height of the optotype character [18, 19]. However, even if tables do not differ in visual angles subtended by optotypes and optotype details, they may significantly differ in visual acuity thresholds. It has been reported that repeatability of measurements of visual acuity made with different charts was poor, with some subjects displaying discrepancies of two lines or more on repeated testing [16, 20-23]. We should consider the definition of “visual acuity” in order to determine why a difference in optotype shape and selection for visual acuity evaluation criteria cause variations in visual acuity measurements. Visual acuity is usually defined as a measure of spatial resolution of the visual system on which the recognition of shape, structure and orientation of objects in space depends [17]. The recognition process is conventionally divided into the phases of sensation, separation and analysis of pattern features, synthesis of signs into complexes, and pattern identification [1, 9, 17]. In early stages, the perceptual system uses information on the retina, to describe the object in terms of primitive components like lines, edges, and angles. The system uses these components to construct a description of the object. Three types of cells in the visual cortex (simple cells, complex cells and hypercomplex cells) can be distinguished by the features to which they respond, and are referred to as feature detectors. These cortical cells do not function autonomously, but interact with each other through neuronal networks, thus enabling grouping of pattern features into a unit. In later stages, the system compares the description to those of various categories of objects stored in visual memory and selects the best match. The more complicated the configuration of optotypes, the more neuronal structures are involved in the analysis of their shapes and their recognition. It is for this reason that the results of visual acuity measurements using optotypes of different shapes may differ from each other and depend on the maturity and preservation of visual analysis mechanisms. Not only differences in optotype shapes but also the criteria selected for assessing resolution (ISO 8597) cause variation in the results of visual acuity measurements [18, 19, 22]. Minimum cognoscible, the smallest familiar figure that the person being tested can recognize, is the criterion used to assess the visual system capacity for resolution of complex optotypes. This criterion is an integral characteristic of the cortical mechanisms for visual perception. Additional criteria may be used to assess individual mechanisms for pattern structure analysis. These selective criteria include such visual perception threshold characteristics as minimum perceptible, minimum visible, mini¬mum resolvable, minimum separable, minimum discrim¬inable, minimum deformable, etc. It should be noted that these criteria are fundamentally different from each other and characterize the state of various visual acuity mechanisms [14, 15, 17, 20, 21]. Thus, minimum visible characterizes the ability to note the presence of an object; minimum perceptible characterizes the ability to note the presence a threshold contrast; minimum separable or minimum resolvable acuity characterizes the ability to distinguish among the details of the object structure; and minimum perceptible allows assessing the shape of a pattern through its contour. While comparing visual acuity measurements among various charts, it should be taken into account that they may be equal to, larger or smaller than the data taken as the basis for comparison. Landolt rings are the International Standards Organisation (ISO) reference optotype [18]. Examining the features of impairments in visual information processing mechanisms requires application of a set of methods which enables identification of selective impairments in resolution based on various criteria [17]. This approach is especially important for diagnostics of meridional amblyopia. Minimum separable and minimum deformable are the selective criteria most commonly used for diagnostics of meridional amblyopia. Another source of contradictions regarding the incidence of meridional amblyopia in astigmats could be the fact that while creating clusters for analysis of visual acuity, researchers take into account only the type of astigmatism and the direction of refractive asymmetries (direct or inverse astigmatism), but do not take into account vector visual acuity asymmetries in relation to refractive asymmetries. From the point of physiological optics, in direct (i.e., with-the-rule) astigmatism, the eye sees vertical lines more sharply than horizontal lines, whereas inverse (i.e., against-the-rule) astigmatism reverses the situation. Consequently, in with-the-rule astigmatism, the visual acuity should be higher in the horizontal retinal meridian, whereas in against-the-rule astigmatism, in the vertical meridian. However, due to continuous refractive changes during visual system development [24], and, consequently, with changes in adaptation to defocus, meridional visual acuity in the horizontal meridian might be higher than in the vertical meridian in some patients with the same type of astigmatiс refractive error. If one does not take into account that meridional visual acuity is a vector quantity, then, statistical processing for the main study patient sample removes the asymmetries in visual acuity which differ from each other in direction, and the mean value provides wrong evidence of the absence of meridional amblyopia. We can avoid this mistake by splitting each group of patients with the same type of astigmatism into three clusters, with these clusters differing in the direction of asymmetry in meridional visual acuity. Group 1 may have equal visual acuities in the vertical and horizontal meridians, group 2 may have visual acuity in the horizontal meridian better than in the vertical meridian, and group 3 may have visual acuity in the vertical meridian better than in the horizontal meridian. This approach will allow a diagnosis of meridional amblyopia to be clarified not only based on the presence of asymmetry in visual acuity in various retinal meridians, but also based on the direction of asymmetry in visual acuity with respect to the principal astigmatic refraction meridians [25, 26]. We have assessed this hypothesis by comparing visual acuities measured using optotypes of various shapes and meridional selective Landolt visual acuities measured with the help of specially developed software. The purpose of the study was to improve the algorithm for diagnosing meridional amblyopia in patients with hyperopic astigmatism on the basis of assessment of asymmetries in meridional separable visual acuity and refraction as vector quantities. Material and Methods Ninety three patients aged 6 to 12 years, with both mild refractive amblyopia and compound with-the-rule hyperopic astigmatism were included in the study, and underwent examination. The sphercal component of refraction ranged from 0.5D to 1.75D, and the astigmatic component of refraction, from 0.75D to 2.0D. Binocular vision at 5 m was assessed under natural conditions. Integral best-corrected visual acuity was assessed using letters and Landolt C-rings of Shevalev Chart and digits generated by a Hoya chart projector. Visual acuity characteristics were determined for the amblyopic eye, fellow eye and binocularly. Meridional separable visual acuity (MSVA) was measured with Landolt C-rings using the software we have developed. Optotypes were presented on the computer screen at a 5-m distance. Threshold MSVA was measured in the meridians corresponding to principal astigmatism axes under gradual change (with an increment of ±7.0 arc second) in the angular size of the optotype. Mean visual acuity in each meridian was determined based on 4 replicates. Tests were presented monocularly and binocularly on a 15-inch 1600×1200-resolution display. Because separable visual acuity is characterized by angular units (arc deg, arc min and arc sec), a decrease in absolute value corresponds to an increase in visual acuity, and vice versa. A gradual change in the angular size of an optotype allows avoiding errors due to the difference in the step in visual acuity (measured in angular terms) between lines, these errors being characteristic for visual acuity chart measurements. It is the possibility of performing multiple visual acuity measurements for a particular pattern orientation that allows for determining selective and not integral, separable meridional visual acuity values. The study was conducted in accordance with applicable local laws and the principles stated in the Declaration of Helsinki. Statistical analyses were conducted using Statistica 6.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ≤ 0.05 was assumed. Results Table 1 shows mean and standard deviation, minimum and maximum values for visual acuity measurements made with optotypes of different configurations in patients with both refractive amblyopia and astigmatism. We believed that there would be a difference in visual acuity measurements obtained with tests of various configurations, giving indirect evidence of impairments in the mechanisms underlying visual perception.
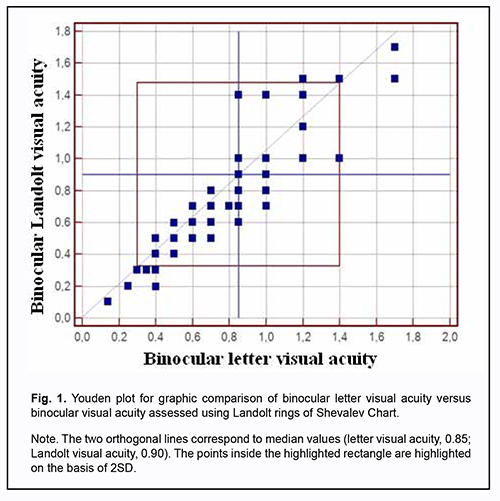
Mean visual acuities measured with Landolt C-rings and otpotypes of various configurations were practically similar, but the difference was not statistically significant. Therefore, a preliminary conclusion could be drawn that the visual acuity measurement methodologies we have used were identical to each other, and the study group was homogeneous. However, because the magnitude of difference between maximum and minimum VA values was substantial, the conclusion was called into question. Graphical analysis of the distribution of Landolt visual acuity across letter visual acuity categories may be used to confirm or disprove this conclusion (Fig. 1). For this purpose, we compared variations in binocular Landolt visual acuity across binocular letter visual acuity categories in 38 patients with both refractive amblyopia and with-the-rule hyperopic astigmatism.
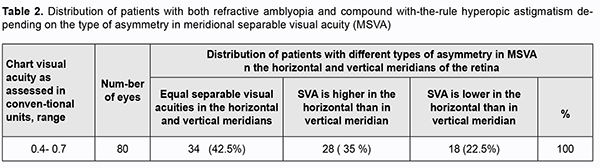
There was a significant variability not only in the magnitude, but also in the sign of difference between binocular Landolt visual acuity and binocular letter visual acuity (Fig. 1), indicating that binocular Landolt visual acuities should be considered as vector quantities. In some patients with binocular letter visual acuity of 0.4 to 1.4, binocular Landolt visual acuity was higher than their binocular letter visual acuity, whereas in other patients of this category, binocular Landolt visual acuity was lower than their binocular letter visual acuity. In patients with binocular letter visual acuity of 0.85 to 1.0, binocular Landolt visual acuity varied from 0.6 to 1.4. In patients with binocular letter visual acuity of 0.4, binocular Landolt visual acuity varied from 0.2 to 0.5, whereas in those with binocular letter visual acuity of 0.7, binocular Landolt visual acuity varied from 0.5 to 0.8. After statistical processing, these asymmetries in Landolt visual acuity become almost negligible, and Landolt visual acuity becomes comparable with letter visual acuity. Variations in the magnitude and sign of visual acuities indicate that Landolt visual acuities should be considered as vector quantities. It should be noted that visual acuities assessed with a conventional Landolt ring chart do not allow reliable distinguishing of meridional differences in visual acuity, because the number of optotypes per line is not sufficient for achieving the level of significance for determining visual acuities in horizontal, vertical and oblique meridians. In addition, sensitivity of visual acuity measurement methods using charts is too low. Steps (of 0.1 of a conventional unit) in visual acuity between lines have discrete values and differ in measurement accuracy from one portion of the operating range to another [17]. Computerized methods of visual acuity testing are helpful in removing these disadvantages. These methods increase study informativeness due to a gradual change in the angular size of the optotype and the possibility of orientating the optotype with respect to a retinal meridian. Tables 2 and 3 show the data related to the type and magnitude of asymmetry in meridional Landolt visual acuity which could indicate heterogeneity of groups of patients with astigmatism.
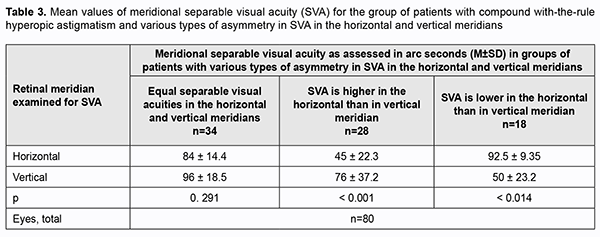
It is well seen (Table 3) that differences in meridional separable visual acuity varied not only in the magnitude, but also in the sign.
Discussion In this study, we used both simple and complex optotype sets (Landolt rings, letters and digits) for comparative analysis of visual acuities in patients with both meridional amblyopia and hyperopic astigmatism. Our analysis took into account that, when using visual stimuli of various configurations, we assess functions of various feature detectors and neuronal structures. It is for this reason that we expected that visual acuity measurements would differ within a group of patients homogeneous with respect to the refractive error. We found that the mean visual acuities were practically similar across the tests. However, significant variations with respect to the mean value supposed that individual visual acuities could differ from test to test. We used Landolt visual acuity values as reference values in order to confirm or reject this hypothesis. The Landolt C is an optotype that is used as the standardized symbol (ISO 8596) for measuring visual acuity and reflects such selective characteristics as minimum visible acuity and minimum separable acuity. The graphic representation of distribution of individual binocular Landolt (minimum separable) visual acuities across various categories of binocular letter (minimum cognoscible) visual acuity demonstrated that Landolt visual acuities are vector quantities and can be higher, lower or equal to letter visual acuities. It should be noted that visual acuities assessed with a conventional Landolt ring chart do not allow reliable distinguishing of meridional differences in visual acuity, because the number of optotypes per line is not sufficient for achieving the level of significance for determining visual acuities in horizontal, vertical and oblique meridians. According to ISO 8566, when testing for visual acuity, the performance level at which the presentation of optotypes shall be terminated is dependent upon the number of optotypes used for each size. Particularly, for a “Pass” assessment: - at least three shall be called correctly if the total number of optotypes used is five; - at least five shall be called correctly if the total number of optotypes used is eight or nine; - at least six shall be called correctly if the total number of optotypes used is ten. We used a computerized method of visual acuity testing to increase informativeness of studying selective meridional visual acuities. The methodology allows to: (1) remove the disadvantages of visual acuity testing associated with discrete in visual acuity between lines in a standard chart, (2) implement multiple visual acuity measurements in various meridians, and (3) determine selective indices specified in angular units. The use of this methodology for assessment of asymmetries in separable visual acuity in orthogonal retinal meridians in astigmats allowed clarifying an algorithm for diagnosing meridional amblyopia. It was found possible to split a homogeneous group of patients with with-the-rule hyperopic astigmatism into three clusters differing in a direction of asymmetry in meridional visual acuity. One cluster had visual acuity in the horizontal meridian better than in the vertical meridian, the second had visual acuity in the horizontal meridian worse than in the vertical meridian, and the third had equal visual acuities in the vertical and horizontal meridians. If one does not take into account that meridional visual acuity is a vector quantity, then, statistical processing for the main study patient sample removes the asymmetries in visual acuity which differ from each other in direction, and the mean value provides wrong evidence of the absence of meridional amblyopia. Variations in vector asymmetries in meridional visual acuity in patients with with-the-rule hyperopic astigmatism indicate that different patients may have different mechanisms of adaptation to astigmatism. In the current study, 42.5% of patients were found to have no meridional amblyopia, and the rest were found to have meridional amblyopia. Particularly, 35% and 22.5% had visual acuity in the horizontal meridian better and worse, respectively, than in the vertical meridian. Application of a vector approach to the assessment of visual acuity in orthogonal retinal meridians in patients with astigmatism allows a diagnosis of meridional amblyopia to be clarified not only based on the presence of asymmetry in visual acuity in various retinal meridians, but also based on the direction of asymmetry in visual acuity with respect to the principal astigmatic refraction meridians. Vector characteristics of meridional visual acuity may be used as an additional criterion in studying adaptation of the sensory system to astigmatism, assessing efficacy of optical correction, and treating meridional amblyopia.
References 1.Sсhiffman HR. [Sensation and perception: An integrated Approach]. 5th ed. St Petersburg: Piтеr; 2003. Russian. 2.Gwiazda J, Bauer J, Thorn F, Held R. Meridional amblyopia does result from astigmatism in early childhood. Clinical Vision Science. 1986;1:145–52. 3.Harvey EM, McGrath ER, Miller JM, et al. A preliminary study of astigmatism and early childhood development. J AAPOS. 2018 Aug;22(4):294-298. doi: 10.1016/j.jaapos.2018.03.004. 4.Hubel DH. Eye, brain, and vision New York: Scientific American Library;1988. 5.Harvey EM, Miller JM, Apple HP, et al. Accommodation in astigmatic children during visual task performance. Invest Ophthalmol Vis Sci. 2014 Aug 7;55(8):5420-30. doi: 10.1167/iovs.14-14400. 6.Harvey EM. Development and Treatment of Astigmatism-Related Amblyopia. Optom Vis Sci. 2009 Jun;86(6):634-9. doi: 10.1097/OPX.0b013e3181a6165f. 7.Harvey EM, Dobson V, Miller JM, Clifford-Donaldson CE. Amblyopia in astigmatic children: Patterns of deficits. Vision Res. 2007 Feb;47(3):315-26. doi: 10.1016/j.visres.2006.11.008. 8.Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt. 34 146–162 10.1111/opo.12123. 9.Vit VV. [The structure of the human visual system] Odessa: Astroprint; 2003. 10.Polat U, Bonneh Y, Ma-Naim T, et al. Spatial interactions in amblyopia: Effects of stimulus parameters and amblyopia type. Vision Res. 2005 May;45(11):1471-9. doi: 10.1016/j.visres.2004.12.014. 11.Freeman RD, Mitchell DE, Millodot M. A Neural Effect of Partial Visual Deprivation in Humans. Science. 1972 Mar 24;175(4028):1384-6. doi: 10.1126/science.175.4028.1384. 12.Mitchell DE, Freeman M, Millodot M, Haegerstrom G. Meridional amblyopia: evidence for modification of the human visual system by early visual experience. Vision Res. 1973 Mar;13(3):535-8. doi: 10.1016/0042-6989(73)90023-0. 13.Dobson V, Miller JM, Harvey EM, Mohan KM, et al. Amblyopia in astigmatic preschool children. Vis Res. 2003 Apr;43(9):1081–90. doi: 10.1016/s0042-6989(03)00014-2. 14.Rozhkova GI, Belozerov AE, Lebedev DS. [Visual acuity measurement: uncertain effect of the low-frequency components of the optotype fourier spectra]. Sensornyie sistemy. 2012;26(2):160-71. Russian. 15.Rozhkova GI. [LogMAR for visual acuity is worse than horsepower for electric lamp]. Sensornyie sistemy. 2017;31(1):29-41. Russian. 16.Colenbrander A. The historical evolution of visual acuity measurement. Vis Impair Res. 2008;10:57–66. 17.Shamshinova AM, Volkov VV. [Functional methods of research in ophthalmology]. Moscow: Meditsina; 1999. Russian. 18.ISO 8596. International Standard. Ophthalmic optics. Visual acuity testing. Standard optotype and its presentation. 2nd edition. Geneve;2009. 19.ISO 8597. International Standard. Optics and optical instruments. Visual acuity testing. Method of correlating optotypes. Geneve; 1994. 20.Bondarko VM, Danilova MV. What spatial frequency do we use to detect the orientation of a Landolt C? Vision Res. 37(15):2153–76. 21.Bondarko VM, Semenov LA. [Acuity and Hyperacuity for Pupils of 11–17 Years Old]. Fiziol Cheloveka. 2012 May-Jun;38(3):56-61. Russian. 22.Graf M, Becker R. Determining visual acuity with LH symbols and Landolt rings. Klin Monbl Augenheilkd. 1999 Aug;215(2):86-90. doi: 10.1055/s-2008-1034677. 23.McGraw P, Winn B, Whitaker D. Reliability of the Snellen chart. BMJ. 1995 Jun 10;310(6993):1481-2. doi: 10.1136/bmj.310.6993.1481. 24.Onufreichuk ON, Rozenblium Yu.Z. [Using vector analysis to study refractive errors in school children]. In: [Ocular biomechanics. Collection of science works]. Moscow; 2005. p.138-43. Russian. 25.Kolomiyets V, Bandura M, Kolomiyets N. [Meridional vernier visual acuity in children and adults with hypermetropic astigmatism]. Oftalmologiia. Vostochania Evropa. 2015; 3(26):27-34. Russian. 26.Kolomiyets V, Bandura M, Kolomiyets N. [Peculiarities of vernier monocular and binocular visual acuity in the retinal orthogonal meridians in patients with hypermetropic astigmatism]. ScienceRise. 2015; 6/4(11):38-44. Russian. DOI: 10.15587/2313-8416.2015.45310
The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|