J.ophthalmol.(Ukraine).2020;6:44-54.
|
http://doi.org/10.31288/oftalmolzh202064454 Received: 18 September 2020; Published on-line: 21 December 2020 Changes in endothelial nitric oxide synthase activity over time after induction of experimental diabetic retinopathy Ya.V. Sirman1, I.V. Savytskyi2 1 Ukrainian Research Institute of Transport Medicine of the Ministry of Health of Ukraine; Odesa (Ukraine) 2 Odesa International Medical University; Odesa (Ukraine) E-mail: farmakod@ukr.net TO CITE THIS ARTICLE: Sirman YaV, Savytskyi IV. Changes in endothelial nitric oxide synthase activity over time after induction of experimental diabetic retinopathy. J.ophthalmol.(Ukraine).2020;6:44-54. http://doi.org/10.31288/oftalmolzh202064454 Purpose: To examine changes in the activity of endothelial nitric oxide (NO) synthase in the development of endothelial dysfunction in experimental diabetic retinopathy corrected by various methods. Material and Methods: Albino (Wistar) rats (weight, 180-200 g) were used in this study and divided into 7 groups 60 animals each. Results: We found impaired endothelial function at day 30 of the experiment, and, subsequently, animals exhibited progression of pathological changes. Hypoglycemic agent only caused a mild, but not significant improvement in endothelial dysfunction, and did not allow restoration of normal synthesis of the proper amount of NO. Application of L-arginine and aflibercept in combination with hypoglycemic therapy for diabetic retinopathy led to repair of endothelial dysfunction and contributed to restoration of the physiological pathway for production of NO. Application of aflibercept and bromfenac in combination with hypoglycemic therapy for diabetic retinopathy led to a less pronounced effect than multi-component therapy including L-arginine, and this effect was not durable and significantly decreased by day 180. Application of aflibercept, L-carnitine and bromfenac in combination with hypoglycemic therapy showed more promising results than the above methods: endothelial NO synthase (eNOS) activity increased at time point 1, and continued to increase subsequently, although normal values were not achieved. Conclusion: Aflibercept, L-arginine and citicoline in combination with hypoglycemic therapy was the most effective method among those tested in this study, with eNOS activity not only increasing as early as day 30, but also continuing to increase subsequently (days 60 and 180) and achieving normal values at day 180. Keywords: experimental diabetic retinopathy, endothelial dysfunction, endothelial NO synthase, correction, metformin, aflibercept, L-arginine, citicoline, L-carnitine, bromfenac
Introduction Diabetes mellitus (DM) is still a menacing challenge in the 21st century, and although a wide variety of advanced medications are available to treat type 1 DM (T1DM) and type 2 DM (T2DM), the disease progresses with time [1]. Approximately half of patients with T2DM exhibit signs of vascular abnormality by the time of diagnosing [2]. The American Heart Association has classified T2DM as a major controllable risk factor that can potentially lead to cardiovascular disease [3]. Vascular lesions (microangiopathies and macroangiopathies) are the main pathogenetic components of various disorders involving the cardiovascular system, which is mainly because of vascular endothelium abnormalities [4]. In addition, diabetes mellitus is a pandemic of the 21st century. According to the International Diabetes Federation (IDF), 424.9 million people had diabetes mellitus in 2017, and the disease is a global problem of modern society [5]. Microvascular and macrovascular complications result from the development and progression of endothelial dysfunction, and worsen the prognosis of patients with diabetes [5]. Endothelial dysfunction in diabetes mellitus is manifested by loss of endothelial cell vasomotor, antithrombotic, anti-inflammatory and adhesive functions [6]. Most antidiabetic medications have no endothelial protective effect. Therefore, undoubtedly, there is a need for the development of novel means of correction of diabetes, which would not only have hypoglycemic effect, but also would be capable of improving vasodilatation and other endothelial functions in diabetes, activating endothelial NO-synthase (eNOS) and normalizing nitric oxide production [5]. Endothelial NO synthase catalyzes the production of nitric oxide from L-arginine in endothelial cells. Three isoforms of NO synthase (neuronal, NOS1; macrophage or inducible, iNO, NOS2; and endothelial, NOS3) differ from each other by their location and the way of their activation [7]. Endothelial NO-synthase is a constitutive form of NO-synthase, i.e., it (a) catalyzes continuous synthesis of nitric oxide, (b) is produced in vascular endothelial cells, and (c) regulates the tone of vascular smooth muscle cells [8, 9]. Diabetes mellitus is characterized by an impaired production of nitric oxide. Reduced production of free nitric oxide results in insufficient capillary dilation, vasospasm and dominance of vasoconstriction factors [10, 11 , 12]. Local concentration of NO is the main contributor to its biological effect. Production of small (physiologically required) amounts of nitric oxide is essential for proper regulation of important body functions [13]. In high concentrations of NO, its indirect effects begin to dominate due to its prolonged overproduction, and cause its cytotoxic effects. Impaired NO production and impaired NO bioavailability are not only a risk factor for cardiovascular disorders, but a predictive factor in the progression of the pathological process. A decrease in the level of biologically active NO and a decrease in NO bioavailability are a key phase in the development of endotherlial dysfunction [13]. The purpose of the study was to examine changes in the activity of endothelial NO-synthase in the development of endothelial dysfunction in experimental diabetic retinopathy corrected by various methods. Material and Methods Albino (Wistar) rats (weight, 180-200 g) were used in this study and divided into 7 groups 60 animals each: Group 1, intact animals; Group 2, animals in which diabetic retinopathy was induced without subsequent correction; Group 3, animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia; Group 4, animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia, injection of aflibercept and L-arginine solution; Group 5, animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia, injection of aflibercept and bromfenac; Group 6, animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia, injection of aflibercept, L-carnitine and bromfenac; Group 7, animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia, injection of aflibercept, L-arginine and citicoline. Type 2 diabetes mellitus and diabetic retinopathy were induced in animals by intraperitonal injection of streptozotocin (Sigma, St. Louis, MO) dissolved in 0.1 M sodium citrate buffer pH 4.5 [14]. Mice were fed with a high-fat diet for 28 days [15] followed by a dose of streptozotocin of 55 mg/kg given in two injections. Doses of drugs used Metformin (Merck Santé s.a.s, Lyon, France), a hypoglycemic agent, was administered at a dose of 300 mg/kg given in 0.9% saline daily through the oral syringe attached to an intragastric tube. The NO donor, L-arginine (SIMESTA, China; quality standard: USP32), was administered by intragastrical injection at a dose of 500 mg/kg given in 0.9% saline through the syringe attached to an intragastric tube. The volume of saline injected depended on animal weight and was not more than 1 mL. The agent was injected daily before morning feeding for 10 days [17]. Aflibercept, an anti-VEGF, was administered by subconjunctival injection daily at a dose of 0.08 mL (25 mg/mL) [18]. Bromfenac ophthalmic solution 0.09% was instilled daily [19]. L-carnitine (Sigma) was administered by intragastrical injection at a dose of 25 mg/ 100 g body weight given as aqueous solution through the syringe attached to an intragastric tube [20, 21]. Citicoline was administered by intramuscular injection daily at a dose of 81.8 mg/kg (0.33 mL/kg). Animals were euthanized at particular time points, days 30, 60 and 180 after induction of experimental diabetes mellitus. Animals were euthanized by decapitation under light anesthesia with ethyl ether in compliance with the Decree No.249 of the Ministry of Health of Ukraine dated 01.03.2012 and the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV (with amendments dated 15.12.2009 and 16.10.2012). Blood was collected from the retroorbital venous plexus located in the orbit behind the globe. A puncture was made by rotating a glass pipette with a sharpened tip and capillary tube attached. After the conjunctival sac was punctured at the medial cantus of the eye, between the globe and the orbit, the pipette was introduced at 2-4 mm posterior to the globe. The pipette tip was considered to be placed in the venous plexus when the capillary tube of the pipette was full with blood. Activity of endothelial NO-synthase was determined spectrophotometrically [22]. Statistics Longitudinal comparisons (between time points) and intergroup comparisons of endothelial and inducible NO-synthase activity were evaluated by parametric statistical tests. Data was checked for normality using Pustylnik’s asymmetry and excess test. Data distribution was considered normal when empirical asymmetry and excess values did not exceed threshold values, i.e., Aemp < Atr, Eemp < Etr, where Aemp and Eemp were calculated asymmetry and excess values, whereas asymmetry and excess threshold values were calculated as follows [23]:
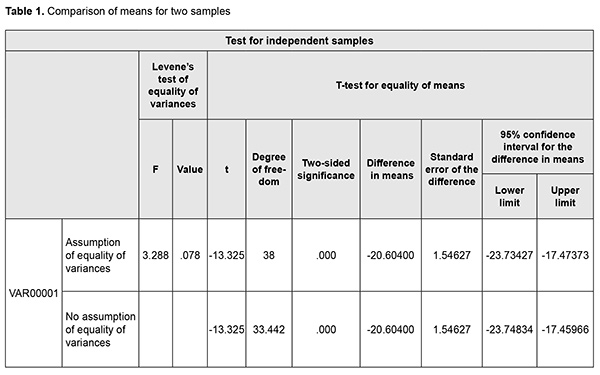
Because all the data was found to be normally distributed, sample mean values were used for further statistical analysis. Independent samples were used for (a) intergroup comparisons and (b) longitudinal comparisons within each group. The statistical hypotheses were as follows: H0: the variance of Group 1 = the variance of Group 2; H1: the variance of Group 1 > the variance of Group 2. Since both hypotheses are directed, we can apply a one-sided test. Hypothesis H0 can be rejected if Femp > Ftr. If the p-value of the test statistic is more than some significance level, the null hypothesis (H0) is accepted; otherwise it is rejected. The level of significance p ≤ 0.05 was assumed. The parametric Student t test was used for comparisons of means. For comparisons of mean values, the directed hypotheses were as follows: H0: the mean value for Group 1 = the mean value for Group 2; H1: the mean value for Group 1 is larger than the mean value for Group 2. In order to make a decision, an absolute value of calculated t was compared with a one-sided threshold value. If |temp| < |ttr|, the null hypothesis could not be rejected. The conclusions could be also made based on the p-value. Statistical analyses were conducted with PASW statistics 18 (SPSS Inc., Chicago, IL) software. We applied the t-test procedure for independent samples which includes comparisons both for variances and mean values. Let us test the hypothesis of homoscedasticity of variances and equality of mean values for animals of group 1 and group 2 at time point 1 with regard to von Willebrand factor (Table 1). PASW statistics 18 software uses Levene's test to assess the equality of variances. Since 0.078 > 0.05, the variances were homoscedastic. Correspondingly, to assess the equality of mean values, values of the first series with the equality of variances should be used. In this series, we had a two-sided p-value of 0, which was less than the selected level of significance (0.05). In subsequent comparisons, if a two-sided p-value was not 0, it was divided by 2 to obtain a one-sided p-value, because we were interested in a directed hypothesis. Therefore, in this example, the mean value of von Willebrand factor for Group 2 was larger than that for Group 1 at time point 1. In subsequent tests, we noted whether the mean values were equal or not, and, if they did not, we had to specify the difference between them. The t-test determines whether the mean values are equal or not, but it does not allow assessing the difference between the mean values accurately. It should be mentioned that this difference is rather relative. We calculated the percentage difference between mean values for Group 1 and Group 2: Therefore, we demonstrated the comparison in mean von Willebrand factor between groups of animals. The Mann-Whitney test was used to determine the statistical significance of differences in particular parameters, with box plots used to show distribution of parameter levels for the groups (Figs 1-3). The level of significance p < 0.05 was assumed.
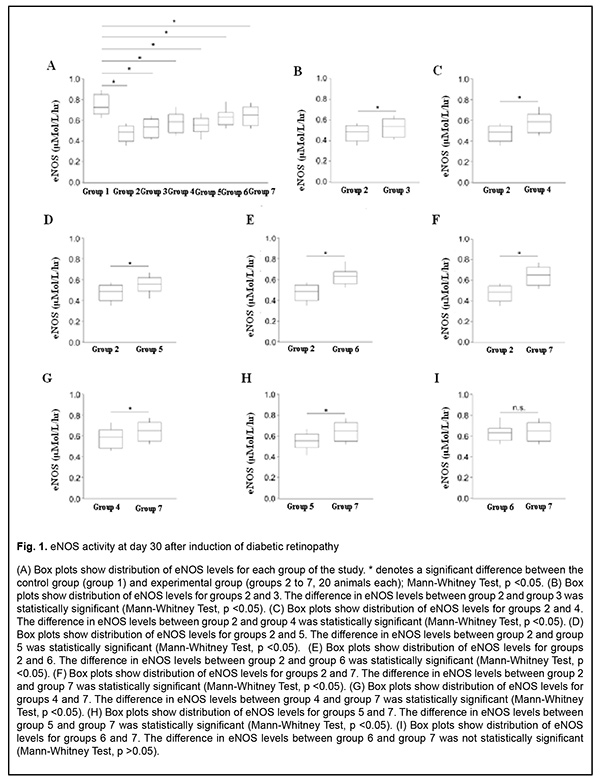
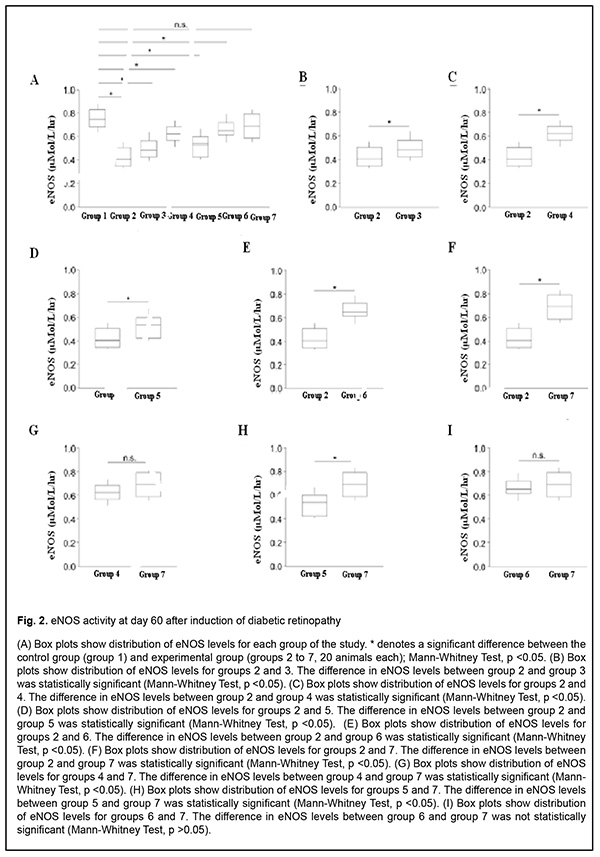
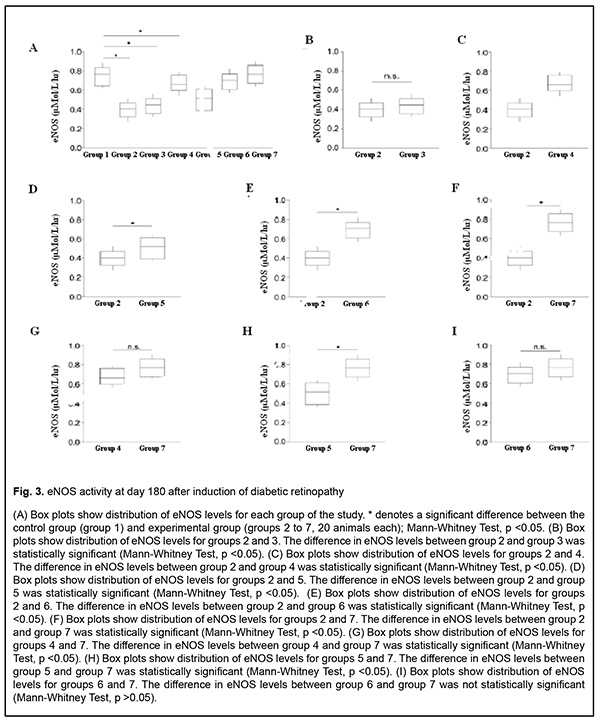
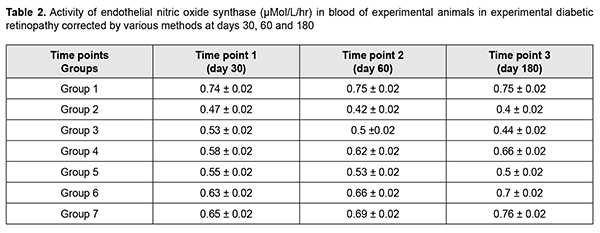
Results The results of our study of changes in activity of endothelial NO-synthase are shown in Table 2 and Figs 1-3.
There was a significant decrease (by 58.07%) in eNOS activity in group 2 (animals in which diabetic retinopathy was induced without subsequent correction) compared to group 1 (intact animals) as early as at time point 1 (р < 0.05). In addition, at time point 2, eNOS activity in group 2 was significantly decreased, by 76.45% (р < 0.05) compared to intact animals, and by 10.95% (р < 0.05) compared to time point 1. Moreover, at time point 3, eNOS activity in group 2 was significantly decreased, by 87.5% (р < 0.05) compared to intact animals, and by 20% (р < 0.05) compared to time point 1, but a decrease compared to time point 2 was not statistically significant. There was a significant decrease (by 41.41%) in eNOS activity in group 3 (animals in which diabetic retinopathy was induced with subsequent correction of hyperglycemia with metformin) compared to intact animals at time point 1 (р < 0.05). However, at this time point, due to positive effect of the hypoglycemic agent, eNOS activity in group 3 was 10.54% higher than in animals in which diabetic retinopathy was induced without subsequent correction (р < 0.05). In addition, at time point 2, eNOS activity in group 3 was significantly decreased, by 51.01% (р < 0.05) compared to group 1, and significantly increased, by 14.42% (р < 0.05), compared to animals in which diabetic retinopathy was induced without subsequent correction, but a decrease compared to time point 1 was not statistically significant. Moreover, at time point 3, eNOS activity in group 3 was significantly decreased, by 68.92% (р < 0.05) compared to intact animals, and significantly increased, by 11.6% (р < 0.05), compared to animals in group 2. At this time point, eNOS activity in group 3 was significantly decreased, by 18.58% (р < 0.05) compared to time point 1, and by 11.71% (р < 0.05) compared to time point 2. In animals of group 4, L-arginine solution and aflibercept were used in addition to the hypoglycemic agent for correction of endothelial dysfunction. At time point 1, eNOS activity in this group was significantly decreased, by 28.81% (р < 0.05), compared to intact animals, and significantly increased, by 18.51% (р < 0.05), compared to group 2 (no correction of hyperglycemia), and by 8.91% (р < 0.05), compared to group 3 (correction of hyperglycemia with metformin). In addition, at time point 2, eNOS activity in group 4 was significantly decreased, by 21.20% (i.e., less apparently than at time point 1) (р < 0.05), compared to intact animals, but there was no significant difference in eNOS activity between time point 2 and time point 1 for group 4. Moreover, at time point 2, eNOS activity in group 4 was significantly increased, by 31.31% (р < 0.05), compared to group 2, and, by 19.74% (р < 0.05), compared to group 3. At time point 3 (day 180), a decrease in eNOS activity in group 4 (12.95%; р<0.05) compared to intact animals was less pronounced than at time point 2. In addition, eNOS activity in group 4 was significantly increased, by 40.89% (р < 0.05), compared to group 2 (with a pathologically decreased eNOS activity), and by 33.13% (р < 0.05), compared to group 3. At this time point, eNOS activity in group 4 was significantly increased, by 12.95% (р < 0.05) compared to time point 1, and by 6.93% (р < 0.05) compared to time point 2. In rats of group 5, diabetic retinopathy was induced with subsequent correction of hyperglycemia, injection of aflibercept and bromfenac. At time point 1, eNOS activity in group 5 was significantly decreased, by 34.51% (р < 0.05), compared to intact animals, and significantly increased, by 14.91% (р < 0.05), compared to group 2. There was, however, no significant difference in eNOS activity between group 5 and group 3 or group 4 at this time point. At time point 2, eNOS activity in group 5 was significantly decreased, by 42.26% (р < 0.05), compared to group 1, but there was no significant difference in eNOS activity between time point 1 and time point 2 for group 5. In addition, eNOS activity in group 5 was significantly increased, by 19.37% (р < 0.05), compared to group 2, and significantly decreased, by 17.38% (р < 0.05), compared to group 4, but there was no significant difference in eNOS activity between group 5 and group 3. At time point 3, eNOS activity in group 5 was significantly decreased, by 50.3% (р < 0.05), compared to intact animals, and significantly increased, by 21.34% and 11.02% (р < 0.05), compared to group 2 and group 3, respectively. Of note that eNOS activity in group 5 was significantly decreased, by 33.07% (р < 0.05), compared to group 4 (correction of hyperglycemia with metformin, L-arginine solution and aflibercept). At time point 3, eNOS activity in group 5 was significantly increased, by 10.92% (р < 0.05) compared to time point 1, but there was no significant difference in eNOS activity between time point 3 and time point 2 for group 5. In animals of group 6, injection of aflibercept, L-carnitine and bromfenac were used in addition to metformin for correction of induced diabetic retinopathy. At time point 1, eNOS activity in group 6 was significantly decreased, by 18.93% (р < 0.05), compared to intact animals, but this decrease was substantially less pronounced than decreases in other groups at this time point. In addition, eNOS activity in group 6 was significantly increased, by 24.76% (р < 0.05), compared to group 2 (no correction for induced diabetic retinopathy), by 15.89% (р < 0.05), compared to group 3, by 7.67% (р < 0.05), compared to group 4, and by 11.58% (р < 0.05), compared to group 5. At time point 2 (day 60), eNOS activity in group 6 was significantly decreased, by 13.06% (р < 0.05), compared to intact animals, but this decrease was less pronounced than at the previous time point, and there was no significant difference in eNOS activity between time point 1 and time point 2 for group 6. Moreover, at this time point, eNOS activity in group 6 was significantly increased, by 35.92% (р < 0.05), compared to group 2, by 25.13% (р < 0.05), compared to group 3, and by 6.72% (р < 0.05) and 20.53% (р < 0.05), compared to group 4 and group 5, respectively. At time point 3 (day 180), eNOS activity in group 6 was significantly decreased, by 7.76% (р < 0.05), compared to intact animals, and this decrease was less pronounced than at time point 2. In addition, at this time point, eNOS activity in group 6 was significantly increased, by 43.61% (р < 0.05), compared to group 2, by 36.21% (р < 0.05), compared to group 3, and by 28.3% (р < 0.05), compared to group 5; it was increased, compared to group 4, but the difference was not statistically significant. At time point 3, eNOS activity in group 6 was significantly increased, by 10.06% (р < 0.05) compared to time point 1, but there was no significant difference in eNOS activity between time point 3 and time point 2 for group 6. In group 7, injection of aflibercept, L-arginine solution and citicoline, in addition to metformin, were used for correction of induced diabetic retinopathy. In this group, positive changes in eNOS activity were observed as early as time point 1, and the level was decreased just by 15.6% compared to intact animals, but was higher than in all other groups at this time point. In addition, at this time point, eNOS activity in group 7 was significantly increased, by 27.15% (р < 0.05), compared to group 2, by 18.56% (р < 0.05), compared to group 3, by 10.6% (р < 0.05), compared to group 4, and by 14.39% (р < 0.05), compared to group 5, but there was no significant difference in eNOS activity between group 6 and group 7. At time point 2, a positive tendency of restoration of eNOS activity in group 7 was still observed, and the level was decreased just by 8.95% compared to intact animals, and was significantly decreased, by 38.25% (р < 0.05), compared to group 2, by 27.85% (р < 0.05), compared to group 3, by 10.11% (р < 0.05), compared to group 4, and by 24.42% (р < 0.05), compared to group 5. There was, however, no significant difference in eNOS activity between group 6 and group 7 at this time point. At time point 3 (day 180), there was no significant difference in eNOS activity between group 7 and intact animals, which confirmed that the best efficacy of this approach to correction of eNOS activity was most pronounced at late time points. In addition, at this time point, eNOS activity in group 7 was significantly increased, by 48.36% (р < 0.05), compared to group 2, by 41.58% (р < 0.05), compared to group 3, by 12.63% (р < 0.05), compared to group 4, by 34.34% (р < 0.05), compared to group 5, and by 8.42% (р < 0.05), compared to group 6. Moreover, at time point 3, eNOS activity in group 7 was significantly increased, by 14.93% (р < 0.05), compared to time point 1, and by 9.54% (р < 0.05), compared to time point 2. Discussion Nitric oxide plays key role in the regulation of endothelial function and vascular tone in patients with diabetes mellitus [24]. Endothelial nitric oxide synthase is an enzyme that transforms NO donor, L-arginine, into NO and citrulline (the physiological pathway of NO production) [25]. Experimental studies have demonstrated that prolonged inhibition of eNOS led to organic sequelae such as severe and prolonged arterial hypertension (AH) with atherosclerosis and organic vascular lesions [26-28]. Evidence of NO involvement in blood pressure regulation has been reported and NO deficiency or rapid NO breakdown in endothelial dysfunction was shown to result in arterial hypertension and cardiovascular disorders [29]. Nitric oxide is a regulator of major endothelial functions, plays a key role in relaxation and decreased migration and proliferation of vascular smooth muscle cells, inhibition of platelet and white cell adhesion to the endothelium, and inhibition of oxidation of low-density lipoproteins [26, 30-32]. NO formation occurs via NO synthase, a Ca2+/calmodulin-dependent enzyme, by oxidation of the guanidine nitrogen of L-arginine to L-citrulline with NO as a by-product [33]. In the current study, there was evidence of endothelial dysfunction and low physiological production of NO as early as day 30 of induced diabetic retinopathy, with progression of pathologic changes at day 60 and especially 180, which was indicated by decreased activity of endothelial NO synthase in group 2 (р < 0.05). The results of group 3 showed that, the hypoglycemic agent only caused a mild, but not significant improvement in endothelial dysfunction, and did not allow restoration of normal synthesis of the proper amount of NO, which confirmed once more that additional means (aside from hypoglycemic agents) should be used for these purposes. The results of group 4 showed that, application of L-arginine and aflibercept in combination with hypoglycemic therapy for diabetic retinopathy led to repair of endothelial dysfunction and contributed to restoration of the physiological pathway for production of NO, with the most pronounced effect observed at day 180, but normal values were not achieved. Application of aflibercept and bromfenac in combination with hypoglycemic therapy for diabetic retinopathy (in animals of group 5) led to a less pronounced effect than multi-component therapy including L-arginine, and this effect was not durable and significantly decreased by day 180. Application of aflibercept, L-carnitine and bromfenac in combination with hypoglycemic therapy in experimental diabetic retinopathy (in animals of group 6) showed more promising results than the above methods. These results indicated that eNOS activity increased as early as time point 1, and continued to increase subsequently, although normal values were not achieved. We found that aflibercept, L-arginine and citicoline in combination with hypoglycemic therapy was the most effective method among those tested in this study, with eNOS activity not only increasing as early as day 30, but also continuing to increase subsequently (days 60 and 180) and achieving normal values at day 180. References 1.Gozhenko AI, Kuznetsova GS, Kuznetsova KS, Byts TM, Susla AB. [Endothelial dysfunction in the pathogenesis of complications of diabetes mellitus. Report 1. Endothelial dysfunction: etiology, pathogenesis and diagnostic methods]. Endokrynologiia. 2017;22(2):171-81. 2.Sukhareva OIu, Shestakova MV. [Current standards and recommendations for therapy for type 2 diabetes mellitus: with a focus on metformin]. Consilium medicum. 2009;11(12):18-24. 3.Steinmetz A, Fenselau S, Schrezenmeir Y. Treatment of dyslipoproteinemia in the metabolic syndrome. Exp Clin Endocrinol Diabetes. 2001;109(4):S548-59. 4.Kuznetsova ES, Kuznetsova AS, Shukhtin VV, Gozhenko AI. [Features of liver autoregulation in type 2 diabetes mellitus]. Ukrainskyi zhurnal nefrologii i dializu. 2015;4(49):21-6. 5.Kurkin DV, Logvinova EO, Bakulin DA, Volotova EV, Tiurenkov IN. [Endothelial protection by GPR119, a novel receptor target in animals with chronic cerebral circulation insufficiency and experimental diabetes mellitus]. Sovremennyie problemy nauki i obrazovaniia. 2018;4. URL: http://www.science-education.ru/ru/article/view?id=27935. (Date of access: 06.09.2020). 6.Van Sloten TT. Vascular dysfunction: At the heart of cardiovascular disease, cognitive impairment and depressive symptoms. Artery Res. 2017;19:18–23. 7.Balabolkin MI, Nikishova MS, Nedosugova LV, Beloiartseva MF, Volkova AK. [Plasma nitric oxide levels in type 2 diabetes mellitus in the presence of treatment with diquertin and tanakan]. Sakharnyi diabet. 2004;1:16-7. 8.Reutov VP, Sorokina EG. [NO-synthase and nitritreductase components of the nitric oxide cycle]. Biochimiia. 1998;63(7):1029-40. 9.Violi F, Marino MT, Milite L. Loffredo L. Nitric oxide and its role in lipid peroxidation. Diabetes Metab Res Rev. Jul-Aug 1999; 15(4):283-8. 10.Arkhipova MM, Neroev VV, Baratova LL, Lysenko VS. [L-arginine in the lacrimal fluid of patients with diabetic retinopathy and possible role of nitric oxide in the pathogenesis of retinal ischemia]. Vestn Oftalmol. Jan-Feb 2000;116(2):23-4. Russian. 11.Arkhipova MM, Vanin AF. [Pathogenetic principles of the therapy of retinal ischemia in vascular diseases of the fundus oculi based on studies of the role of nitrogen oxide]. Vestn Oftalmol. Jan-Feb 2001;117(1):51-3. Russian. 12.Donati G, Pournaras C. Endogenous deficiency of Nitric Oxide is an aggravating factor in retinal vein occlusion. Klin Monbl Augenheilkd. 1998 May;212(5):324-5. 13.Dzugkoev SG, Dzugkoeva FS, Metelskaia VA. [Role of nitric oxide in the formation of endothelial dysfunction in diabetes mellitus]. Kardiovaskuliarnaia terapiia i profilaktika. 2010;9(8):63-8. Russian. 14.Pasyechnikova NV, Moroz OA. [Protective effect of quercetin and lypoate of functional groups on proteins in retina with experimental diabetes]. Oftalmol Zh. 2015;3:76-81. 15.Kaidash OA, Ivanov VV, Vengerovskii AI, Buiko EE, Shchepetkin IA. [Murine diabetes mellitus model induced by high‐fat diet and low‐dose streptozotocin]. Biulleten’ sibirskoi meditsiny. 2020;19(2):41-7. Russian. 16.Bairasheva VK, Babenko AYu, Dmitriev YuV, Bairamov AA, Chefu SG, Shatalov IS, Aref’ieva AN, Pchelin IYu, Khudiakova NV, Aliev PG, Grineva EN. [Role of metformin in the prevention of diabetic neuropathy in experimental type 2 diabetes mellitus]. Regionarnoie krovoobrashchenie i mikrotsirkuliatsia. 2016;15(3):70-80. Russian. 17.Prokrovskii MV, Prokrovskaia TG, Korchakov VI, Artiushkova EB. [Endothelioprotective properties of L-arginine in a nitric oxide deficiency model]. Eksp Klin Farmakol. Mar-Apr 2008;71(2):29-31. Russian. 18.Gal-Or O, Livny E, Sella R, Nisgav Y, Weinberger D, Livnat T, et al. Efficacy of subconjunctival aflibercept versus bevacizumab for prevention of corneal neovascularization in a rat model. Cornea. 2016 Jul;35(7):991–6. 19.Pavlova ON, Gulenko ON, Karimova EG, et al. [Changes in serum catalase activity over time after mechanical effect on the blood-retinal barrier]. Mezhdunarodnyi nauchno-issledovatelskii zhurnal. 2020;5(95) Part 1:153–8. Russian. 20.Bykov IL. [Effect of L-carnitine on metabolic disorders in rats with experimental acyl-CoA dehydrogenase deficiency]. Eksp Klin Farmakol. Nov-Dec 2004;67(6):48-52. Russian. 21.Dzugkoev SG, Dzugkoeva FS, Gumanova NV, Metelskaia VA. [Effect of coenzyme Q10, afobazole and L-carnitine on endothelial function in rats with experimental diabetes mellitus. Kubanskyi nauchnyi meditsinskii vestnik. 2012;3(132):48-51. Russian. 22.Kovaliova OM, Demidenko GV, Gorbach TV. [Diagnosis of endothelial function: assessment of a vasoactive nitric oxide pool]. Ministry of Health of Ukraine. Ukrainian center for medical science information and patent and license activity. Kyiv: SPD- FO Tarasenko; 2006. Russian. 23.Lupan IV, Avramenko OV, Akbash KS. [Computer Statistical Packages: teaching aid]. Kirovohrad: KOD; 2015. Ukrainian. 24.Monisha B, Vats P. Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. Indian J Hum Genet. 2014 Jan;20(1):10-9. 25.Reutov VP, Sorokina EG, Okhotin VE, Kositsyn NS. [Cyclic nitric oxide transformations in the body of mammals]. Moscow:Nauka; 1988. Russian. 26.Yarmysh NV, Grozna LN. [Endothelial dysfunction and its regulatory factors]. Visnyk problem biolohiyi ta medytsyny. 2014;3:37–43. Russian. 27.Casas JP, Cavalleri GL, Bautista LE, et al. Endothelial nitric oxide syntase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol. 2006 Nov 15;164(10):921-35. 28.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007 Jan;56(1):118-26. 29.Kuzminova NV, Serkova VK. [Vascular endothelial function in patients with essential hypertension]. Ukrainskyi terapevtychnyi zhurnal. 2008;2:21-7. 30.Corretti MC, Anderson TJ, Beniamin FJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Amer Coll Cardiol. 2002 Jan 16;39(2):257-65. 31.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002 Mar 23;359(9311):995-1003. 32.Garaliene V. Endothelium and nitric oxide. Medicina (Kaunas). 2008;44(7):564-9. 33.Rippe C, Lesniewski L, Connell M, et al. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010 Jun;9(3):304-12. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|