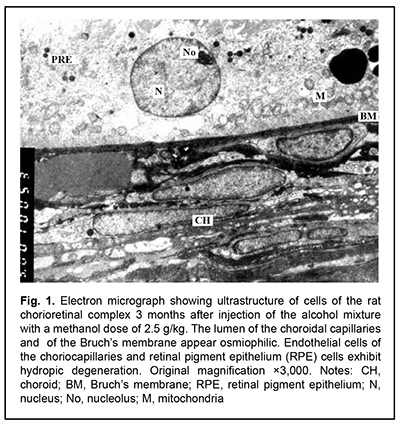
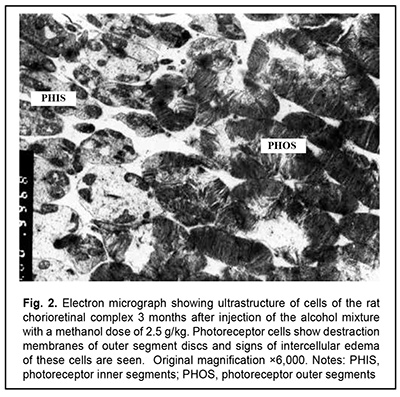
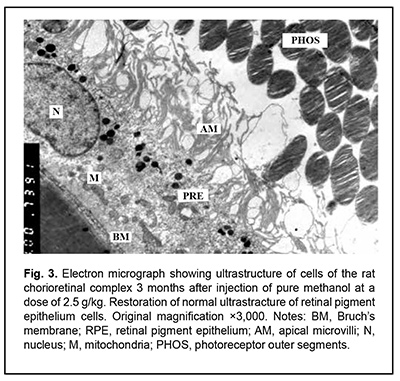
J.ophthalmol.(Ukraine).2020;6:25-29.
|
http://doi.org/10.31288/oftalmolzh202062529 Received: 12 June 2020; Published on-line: 21 December 2020 Late ultrastructural changes in the rat chorioretinal complex following injection of mixture of 40% ethanol and 100% methanol N. I. Molchaniuk SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: elmicroscop@gmail.com TO CITE THIS ARTICLE: Molchaniuk NI. Late ultrastructural changes in the rat chorioretinal complex following injection of mixture of 40% ethanol and 100% methanol. J.ophthalmol.(Ukraine).2020;6:25-9. http://doi.org/10.31288/oftalmolzh202062529 Background: Consumption of surrogate alcohol containing methanol may result in blindness and even death. The literature is scant on experimental and especially morphological studies on the effect of surrogate alcohol containing methanol and ethanol on organs (particularly, the eye) and tissues of experimental animals. Purpose: To examine late ultrastructural changes in the interplay of cells of the rat chorioretinal complex (endothelial cells of the choriocapillaries, retinal pigment epithelium (RPE) cells, and photoreceptor cells) following a single intraperitoneal (IP) injection of alcohol mixture (40% ethanol and 100% methanol) at the proportion of 3:1, with a methanol dose of 2.5 g/kg. Material and Methods: Twelve adult Wistar rats (weight, 250-300 g) were divided into two groups, each of 6 rabbits. Group 1 (the experimental group) received a single IP injection of alcohol mixture (40% ethanol and 100% methanol) at the proportion of 3:1, with a methanol dose of 2.5 g/kg, whereas group 2 (controls) received a single IP injection of 100% methanol at a dose of 2.5 g/kg. The LD50 value for IP administration of methanol in rats is reported as 9.5 g/kg body weight. The ultrastructure of endothelial cells of the choriocapillaries, RPE cells, and photoreceptor cells was examined on a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine) at 1 and 3 months after IP injection of the above alcohol mixture in rats. Results: At one month after IP injection of the alcohol mixture, the lumen of the choroidal capillaries and ground substance of the Bruch’s membrane appeared osmiophilic, indicating increased lipid levels. In most choroidal capillaries, endothelial cells exhibited signs of hydropic degeneration. The RPE cells showed polymorphic changes; some of them showed severe degeneration of organelles, sometimes with total loss of cytoplasm and damage to the plasmalemma at the basal and apical surfaces; some other RPE cells showed signs of compensatory and restorative processes aimed at intracellular repair processes. There were signs of intercellular and intracellular edema and degeneration of membrane structure in the photoreceptor cell layer. At 3 months after IP injection of the alcohol mixture, signs of hydropic degeneration in the examined structures of the chorioretinal complex were somewhat less severe than at the previous time point. A long-term toxic effect of consuming a small dose of methanol was characterized by severe pathologic changes in and poor reserve potential of the cells of the chorioretinal complex, which was reflected in slow repair processes during the period from 1-month to 3-month time points. This likely explains the reported cases of a prolonged serious condition of individuals after consuming surrogate alcohol. Conclusion: A single IP injection of the alcohol mixture with a methanol dose of 2.5 g/kg body weight resulted both in signs of hydropic degeneration and slow repair processes in the cells of the rat chorioretinal complex during the period from 1-month to 3-month time points. A single IP injection of pure methanol (2.5 g/kg) resulted in uniform and more severe changes in the cells of the rat chorioretinal complex. Methanol can be attributed a leading role in the development of pathological changes in the examined structures of the chorioretinal complex after injection of the alcohol mixture. Keywords: ultrastructure, degenerative changes in the cells of the chorioretinal complex, choriocapillaries, retinal pigment epithelium, toxic effect of ethanol and methanol mixture
Introduction Penetrating globe injury is a leading cause of registered visual disability among working-age adults. Intraocular foreign bodies (IOFBs) account for 18%-41% of all penetrating globe injuries. The visual prognosis of an injury with an IOFB is determined by the size, composition, depth and location of the foreign body, and its duration in situ [1-3]. Endophthalmitis is one of the most severe potential complications of penetrating globe injury. Among patients with infectious endophthalmitis, post-traumatic endophthalmitis comprises about 10–30% of cases. Factors associated with increased risk of endophthalmitis following penetrating trauma include retained IOFB, delay in wound closure of >24 h and injury in a rural setting. Soil contamination is believed to result in a higher rate of endophthalmitis for open globe injuries occurring in a rural versus a non-rural setting. Endophthalmitis developed in 30% of patients with rural penetrating trauma, compared with 11% of patients with non-rural penetrating trauma [4, 5]. The incidence of endophthalmitis was higher after IOFB-related penetrating globe injury than after penetrating globe injury without IOFB [6]. Metallosis is a dangerous complication after metallic IOFB-related penetrating globe injury. It occurs through damage to intraocular tissue through toxic effects of retained metallic IOFB, these effects manifesting clinically as recurrent episodes of choroidal inflammation. Clinical manifestations developing as early as the first weeks after the traumatic event and cases of metallic foreign bodies presenting years after the initial trauma without causing severe ocular inflammatory response have been rarely reported [7-11]. Therefore, early detection of an IOFB is not only important for selecting the most appropriate surgical treatment strategy but also a factor influencing the treatment outcome. Despite continuous advances in imaging techniques, early detection of an IOFB is still a challenge for the ophthalmologist. Visualizing a ciliary body IOFB is especially challenging. Approximately 5% of IOFBs are found in the projection of or close to the ciliary body [3, 7]. Computed tomography (CT), radiography with the Komberg-Baltin prosthesis, ultrasonography and magnetic resonance imaging (MRI) are the most effective techniques for detecting and localizing an IOFB. Nevertheless, there are an ample number of reports of missed anterior chamber IOFBs [8, 10]. We have previously demonstrated that near-infrared transpalpebral transillumination (NIR TPT) is a modality enabling easy and non-invasive visualization and assessment of the sizes of ciliary body structures [11-13]. In addition, NIR TPT enables visualization of shadows of ciliary body IOFBs of various origin (metallic, stone and wood) in patients with penetrating globe injuries. The use of NIR TPT as means for visualizing an IOFB in conjunction with traditional imaging modalities seems promising and would improve our ability to diagnose these patients preoperatively [14-16]. The purpose of the study was to compare detection rates of near-infrared transpalpebral transillumination, ultrasonography and radiography for foreign bodies situated in the anterior segment of the eye. Material and Methods This was an open-label, prospective and non-interventional study. This study was performed within the framework of a planned research design and the study protocol was approved by the Bioethics Committee of the Filatov Institute. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Informed consent was obtained from all study subjects. Thirty male patients (30 eyes; age, 21 to 65 years) with penetrating globe injuries and suspected foreign body in the anterior segment of the eye (the anterior chamber, lens, anterior vitreous cavity) were under our observation. They underwent visual acuity assessment, biomicroscopy, ophthalmoscopy, metal detector examination, anteroposterior and lateral radiography (both with and without using the Komberg-Baltin prosthesis), ultrasound biometry, ultrasound scanning of the anterior eye and posterior eye; ultrasound distant perimetry; metal detector examination; and NIR TPT. A diagnosis of IOFB was confirmed or denied by the three imaging modalities (NIR TPT, ultrasound scanning and X-ray examination) using the signs that were specific for each of the modalities. The NIR TPT system used consisted of (1) a wireless infrared light-emitting-diode light source with a dominant wavelength of 940 nm and (2) slit-lamp attachable monochrome video camera (Blackfly®, FLIR Integrated Imaging Solutions Inc., Canada) capable of recording NIR images and video [14, 15, 17]. The transpalpebral illumination study was performed with the patient sitting at the slit lamp. Images of scleral shadows of the IOFB, pars plicata and pars plana were taken and saved in the computer. We used the Aviso ultrasound (Quantel Medical; Cournon d'Auvergne, France) with 10-MHz and 50-MHz linear probes for the posterior and anterior segments of the eye, respectively. The ultrasound study was performed with the patient lying on his back, and with the head of the bed raised. In the ultrasound study with the anterior probe, patients were administered topical anesthesias with ophthalmic 0.5% proparacaine hydrochloride (ALCAINE®, SA Alcon-Couvreur NV, Puurs, Belgium), and, subsequently, Vidisic Eye Gel (Dr. GERHARD MANN Chem.-Pharm. Fabrik, Berlin, Germany) as a contact gel. The posterior probe was placed over the patient’s closed eyelid to scan the eye non-invasively. Targeted anteroposterior and lateral radiography (with the Komberg-Baltin prosthesis) was performed using a Proteus XR/a radiography system (GE Healthcare, Milwaukee, Wis), and patients were administered topical anesthesias with ophthalmic 0.5% proparacaine hydrochloride (ALCAINE®). Contact invasive imaging studies (radiography with the Komberg-Baltin prosthesis, ultrasound scanning of the anterior eye and ultrasound biometry) were not performed in patients with a non-adapted corneal or scleral wound. Receiver operating characteristic (ROC) curves were constructed to obtain graphical representation of selectivity and specificity, and the area under the ROC curve was calculated to compare the efficacy of various imaging techniques for detection of foreign bodies in the anterior eye. Predictive characteristics for the techniques for the detection of IOFBs are reported as mean and 95% confidence intervals (CI). Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software [18]. Results An intraocular foreign body was detected in the anterior segment (the anterior chamber, lens, anterior vitreous cavity) in all the 30 patients. Of the 30 eyes with IOFB, a foreign body was identified and localized by radiography in 21 eyes (70%). Radiography failed to detect a foreign body in the rest 9 eyes (30%) because the foreign body was either composed of organic material (rock or wood) or metallic and small (less than 1.0 mm). Of note that, in 6 patients (20%), a foreign body was found by radiography, but it was not possible to localize it with the Komberg-Baltin prosthesis due to the non-adapted wound of the globe and associated risk of iatrogenic complications. Radiography showed no false positives, but 3 false negatives, all of which were associated with small (≤0.5 x 0.5 mm) metallic foreign bodies. Of the 30 eyes with IOFB, a foreign body was identified by ultrasound in 24 eyes (80%). Ultrasound scanning of the anterior eye could not be performed in 6 patients (20%) due to the non-adapted wound of the globe. Ultrasonography showed no false positives. NIR TPT revealed a foreign body in the anterior eye in 28 eyes (93.3%) of the 30 eyes with IOFB. Particularly, NIR TPT found a foreign body in the anterior segment in 3 eyes in which either an IOFB was missed by radiography and ultrasound, or ultrasound could not be performed. NIR TPT failed to detect a foreign body in 2 eyes (6.6%). In one of these two eyes, a foreign body could not be detected by NIR TPT because of shielding effect of massive subconjunctival hemorrhage, but it was detected both by radiography and ultrasound. In the other eye, neither of the imaging modalities could detect the IOFB because the latter was too small, but the foreign body was revealed during surgery. In addition, two eyes were mistakenly found to have a foreign body in the anterior vitreous (false positive results) by NIR TPT, but this was subsequently confirmed neither by radiography nor by ultrasound. These two patients had subconjunctival and vitreous hemorrhages. Of note is that the method we have developed (1) enabled the diagnosis of IOFB in swollen and opaque lens masses and opaque corneas, and (2) correctly detected not only X-ray positive foreign bodies, but also X-ray-negative foreign bodies. ROC analysis of the IOFB detection methods was performed for 50 eyes; the presence of a foreign body was confirmed for 30, and the absence of a foreign body was confirmed for 20 of these eyes. ROC curves for the three IOFB detection methods are shown in Figure 1.
The area under curve (AUC) for NIR TPT was 0.92 (95% CI, 0.80–0.98); for radiography, 0.82 (95% CI, 0.68–0.91), and for ultrasound, 0.87 (95% CI, 0.74–0.95), demonstrating good efficacy of all the three IOFB detection methods, although there was no significant difference (p > 0.05) in AUC between each two of them. Table 2 shows predictive characteristics of the methods under analysis. Discussion Inadequate foreign body visualization is the main reason of unsuccessful removal attempts and late removal of foreign bodies situated in the anterior segment of the eye [19-21]. Although radiography is an effective imaging modality enabling detection and accurate localization of an IOFB, detecting an X-ray negative foreign body by radiography is difficult. Thus, the foreign bodies composed of organic materials (stone or wood) were missed by radiography in our study. In addition, small (≤ 1.0 mm) metallic foreign bodies may be missed by radiography due to their shape and/or location in the anterior chamber angle, beneath the iris, or in the ciliary body area, which was confirmed by the current study. Moreover, accurate IOFB localization by radiography with the Komberg-Baltin prosthesis sometimes cannot be performed due to the non-adapted wound of the globe and/or associated risk of iatrogenic complications. Ultrasonography is another imaging modality proved to be effective in detecting foreign bodies of various sizes, shapes, materials and locations (including the anterior segment of the eye). In some cases, however, ultrasonography should not be performed due to high risk of iatrogenic complications. Patients with non-adapted corneal or scleral defects may have additional wound infection; prolapse of the retina, choroid or sclera; or iatrogenic expulsive hemorrhage due to globe contact with or compression by the probe that is applied directly upon the surface of the globe [22]. Thus, ultrasound study of the anterior segment of the eye could not be safely performed in 19.8% of study patients due to the presence of a non-adapted globe wound, which limited out ability to diagnose IOFB in these patients. The use of near-infrared LED illumination in NIR TPT enables transillumination not only through the sclera, but even through the patient’s lid. In addition, absence of physical contact between LED light source and the cornea and sclera allows excluding complications and trauma of these ocular structures. We managed to conduct the NIR TPT procedure quickly and safely with as little pain and discomfort to the patient as possible in all cases of the study. Particularly, no iatrogenic damage was observed after performing the procedure in patients with non-adapted corneal and scleral defects. The use of LED as a source of infrared light simplifies the globe transillumination system, and there is no need for the use of optic fibers to transmit the light radiation to the eye. The LED light source is a compact wireless device that needs no additional infrared filters. One may use infrared LEDs of different wavelengths to achieve better visualization of the globe. With the use of near-infrared light for globe transillumination, there is no glare from bright visible light, which makes the conduct of studies in this patient category easier. The proposed visualization approach enables taking real-time photographs and shooting real-time video of TPT pictures [11-13]. In addition, we managed to visualize IOFBs of various compositions and dimensions, particularly, in the presence of opaque media using NIR TPT. Thus, NIR TPT found a foreign body in the anterior eye in the three eyes (10% of the study patients) in which either an IOFB was missed by radiography and ultrasound, or ultrasound could not be performed due to the presence of a non-adapted globe wound. Moreover, NIR TPT enables visualizing ciliary body structures and localizing and measuring the dimensions of shadows cast by these structures on the sclera [14-16]. Thus, in the current study, we managed to localize an IOFB shadow with respect to shadows cast by ciliary body structures on the sclera, which allowed localization of a foreign body and selecting the most appropriate surgical treatment strategy. The method of NIR TPT, however, has certain drawbacks. For example, a foreign body in the projection of the ciliary body is difficult to identify in the presence of massive subconjunctival hemorrhage due to a shielding effect of blood with respect to the near-infrared light. In addition, intensive absorption of near-infrared light by subconjunctival blood in some cases led to incorrect assessment of NIR TPT pictures and false positive results. The use of a non-invasive NIR TPT in conjunction with traditional imaging modalities (radiography and ultrasound) resulted in a ten percent increase in the rate of detection of anterior segment foreign bodies due to the detection of some X-ray-negative IOFBs and identification of small (less than 1 mm) IOFBs.
References 1.Zabrodskiĭ PF. [Immunotoxicological characteristics of methanol. Correction of immune system dysfunction]. Saratov; 2013. 241 р. Russian. 2.Manuchehri AA, Alijanpour E, Daghmechi M, et al. A case of methanol poisoning leading to prolonged respirator dependence with consequent blindness and irreversible brain damage. Caspian J Intern Med. 2015;6 (3):180–3. 3.Rajamani R, Muthuvel A, Senthilvelan M, Sheeladevi R. Oxidative stress induced by methotrexate alone and in the presence of methanol in discrete regions of the rodent brain, retina and optic nerve. Toxicol Lett. 2006 Sep 10;165(3):265-73. 4.Sweeting JN, Siu M, McCallum GP, et al. Species differences in methanol and formic acid pharmacokinetics in mice, rabbits and primates. Toxicol Appl Pharmacol. 2010 Aug 15;247(1):28-35. 5.Zhai R, Zheng N, Rizak J, Hu X. Evidence for Conversion of Methanol to Formaldehyde in Nonhuman Primate Brain. Anal Cell Pathol (Amst). 2016;2016:4598454. 6.Molchaniuk NI. [Light and electron microscopic study of choriocapillaries, pigment epithelium and photoreceptor cells of rat retinas in dynamics after administration of various doses of methanol]. Visnyk Kyivskogo natsional`nogo universitetu imeni Tarasa Shevchenka. Problemy reguliatsii fiziologichnykh funktsii. 2015;1(18):74–8. Ukrainian. 7.Molchaniuk NI. [Ultrastructural changes in the rat choroid and retina after injection of alcohol mixture (40% ethanol and 100% methanol)]. Visnyk Kyivskogo natsional`nogo universitetu imeni Tarasa Shevchenka. Biologiia. 2019;79:16–24. Ukrainian. 8.Zabrodskiĭ PF, Germanchuk VG. [Effect of ethanol on methanol immunotoxicity]. Eksp Klin Farmakol. 2001 Sep-Oct;64(5):40-2. Russian. 9.Pohanka M. Toxicology and the biological role of methanol and ethanol: current view. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016 Mar;160(1):54-63. 10.Akimov PА, Orbidans АG, Terekhin GА, Terekhina NА. The influence of acute alcohol intoxication on glycogen content in the liver and skeletal muscles. Patol Fiziol Eksp Ter. 2010; 2:15-17. Russian. 11.Patra M, Salonen E, Teramaetal E. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Вiophys J. 2006 Feb 15;90(4):1121-35. 12.Golovenko NIa, Larionov VB, Ovcharenko NV, Borisiuk IIu, Lihota ЕB. [Toxic-kinetic interaction of ethyl and methyl alcohol in white mice]. Sovremennyie problemy toksikologii. 2008;(1):32–6. Ukrainian. 13.Molchaniuk NI. [Effect of alcohol mixture (40% ethanol and 100% methanol) on the ultrastructure of the rat choroid and retina]. Visnyk problem biologii i meditsiny. 2019;4(1):222-7. Ukrainian. 14.Molchaniuk NI. [Ultrastructure of rat hepatocytes in the early stages after intraperitoneal methanol injection]. Bukovynskyi medychnyi visnyk. 2013; (4):100–3. Ukrainian. 15.Ostrovskii MA, Feldman TB. [Chemistry and molecular physiology of vision, light-sensitive protein rhodopsin]. Uspekhi khimii. 2012; 81(11):1071-90. Russiаn. 16.Paasma R, Hovda KE, Jacobsen D. Methanol poisoning and long term sequelae - a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol. 2009 Mar 27;9:5. doi: 10.1186/1472-6904-9-5. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|