J.ophthalmol.(Ukraine).2020;6:14-18.
|
http://doi.org/10.31288/oftalmolzh202061418 Received: 18 August 2020; Published on-line: 21 December 2020 Serum TNF-α and IL-1β levels in patients with RNFL thinning in uveitis complicated by optic nerve inflammation M. V. Panchenko, M. M. Nikolaienko, O. M. Honchar, D. O. Prykhodko, H. S. Pereiaslova, O. O. Sokol Kharkiv National Medical University; Kharkiv (Ukraine) E-mail: panchenko0802@gmail.com TO CITE THIS ARTICLE: Panchenko MV, Nikolaienko MM, Honchar OM, Prykhodko DO, Pereiaslova HS, Sokol OO. Serum TNF-α and IL-1β levels in patients with RNFL thinning in uveitis complicated by optic nerve inflammation. J.ophthalmol.(Ukraine).2020;6:14-8.http://doi.org/10.31288/oftalmolzh202061418 Purpose: To assess serum tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) levels in patients with retinal nerve fiber layer (RNFL) thinning in uveitis complicated by optic nerve inflammation. Material and Methods: One hundred and thirty-two patients underwent examination and treatment. Serum TNF-α and IL-1β levels were determined by commercially available enzyme-linked immunoassay kits. Results: The serum TNF-α level was more than 1.5 times higher in patients with uveitis complicated by optic nerve inflammation who had reduced RNFL thickness compared to patients with normal RNFL thickness (p < 0.05), and the serum IL-1β level was 17% higher in the former patients than in the latter patients (p > 0.05). Conclusion: In the active stage of uveitis, the serum TNF-α level was significantly higher in patients with RNFL thinning due to uveitis complicated by optic nerve inflammation compared to patients with normal RNFL thickness, and the serum IL-1β level was higher in the former patients than in the latter patients, but the difference was not statistically significant. The results of the study may justify the use of biologic therapy in the treatment of uveitis complicated by optic nerve inflammation. Keywords: uveitis complicated by optic nerve inflammation, TNF-α, IL-1β
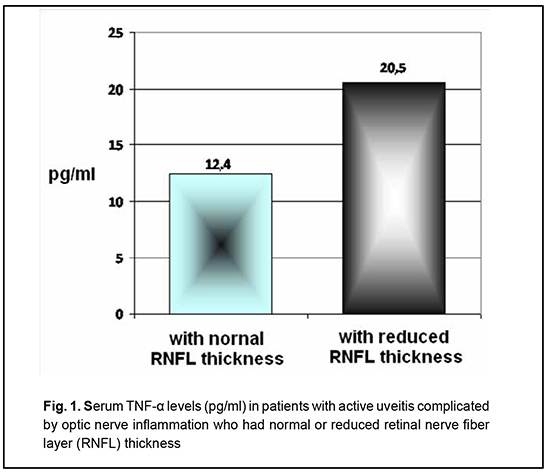
Introduction Tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) are important in the pathogenesis of uveitis, which is indicated by their increased levels in the aqueous humor [1-4], vitreous [5, 6], and serum [7-11] of patients with the disease. Aqueous humor TNF-α [1], serum TNF-α [10] and aqueous humor IL-1β [4] levels have been found to correlate with disease activity in uveitis. However, opinions vary regarding the role of TNF-α and IL-1β in optic nerve injury or lesion. Experimental studies have demonstrated that IL-1β promotes the induction of retinal autoimmune disease such as autoimmune uveoretinitis [12] and is involved in caspase-1-mediated cell death, known as "pyroptosis" [13]. In addition, an increase in TNF-α expression has been correlated with optic nerve injury [14]. Susceptibility to experimental autoimmune uveoretinitis (EAU) and endotoxin-induced uveitis (EIU) in vivo is correlated with the extent of TNF production by retinal Müller glia (RMG) and retinal pigmented epithelium (RPE) cell types under in vitro conditions [15]. Findings of retinal ganglion cell and optic nerve axon death after intravitreal administration of TNF-α have become the basis for a generally recognized experimental model of optic nerve degeneration [16-18]. The role of TNF-α in the development of retinal ganglion cell apoptosis has been demonstrated in the experimental corneal alkali burn model [19]. However, an experimental study by Mac Nair et al [14] found that TNF-α has an early protective effect on retinal ganglion cells after optic nerve crush. Obviously, the question requires further exploration. The purpose of this study was to assess serum TNF-α and IL-1β levels in patients with RNFL thinning in uveitis complicated by optic nerve inflammation. Material and Methods The study protocol complied with the tenets of the Declaration of Helsinki. Inclusion criteria were men and women aged 18 years or younger, with uveitis complicated by optic nerve inflammation. Exclusion criteria were diabetes; acute infections; cardiovascular diseases; abnormal circulation in the major ocular vessels; history of ocular surgery; pregnancy; or breast feeding. One hundred and thirty-two patients (53 men and 79 women) aged 18 to 74 years were involved in the study and underwent examination and treatment for uveitis complicated by optic nerve inflammation. The duration of uveitis ranged from one month to 14 years. Patients underwent a routine eye examination, including, but not limited to, ultrasound biomicroscopy and optical coherence tomography (OCT). Serum TNF-α and IL-1β levels were determined by commercially available enzyme-linked immunoassay kits. Sera of 40 healthy volunteers were used as controls. Statistica 6.1 software was used for the statistical analyses. Significance of differences was determined by Student’s t-test, Pearson’s chi-square or Fisher's Exact test, as appropriate. Mean values are presented as the mean ± standard error. Results The current and our previous studies [20] found that the serum TNF-α level in patients with active uveitis complicated by optic nerve inflammation was significantly increased compared with healthy donors (15.8 ± 1.02 pg/ml and 4.1 ± 1.52 pg/ml, respectively, p < 0.05). In addition, the serum TNF-α level in patients with RNFL thinning due to uveitis complicated by optic nerve inflammation (Fig. 1) was 1.5 times higher compared to patients with normal RNFL thickness (20.5 ± 1.24 pg/ml vs 12.4 ± 1.47 pg/ml, respectively, p < 0.05).
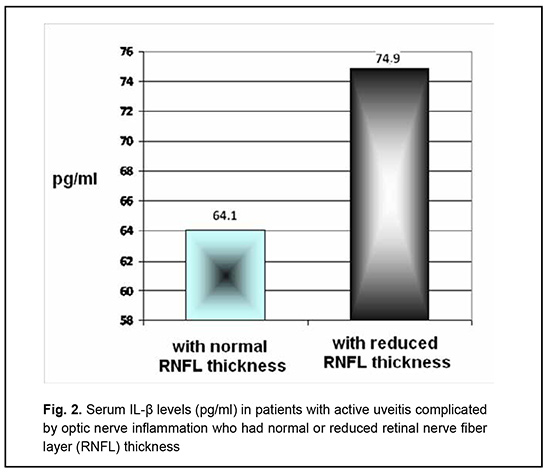
The serum IL-1β level in patients with active uveitis complicated by optic nerve inflammation was significantly increased compared with healthy donors (67.3 ± 5.24 pg/ml vs 35.6 ± 4.1 pg/ml, respectively, p < 0.05). Fig. 2 shows serum IL-1β levels in patients with uveitis complicated by optic nerve inflammation who had not vs had RNFL thinning. The serum IL-1β level in patients with active uveitis complicated by optic nerve inflammation who had RNFL thinning was 17% higher than in those who had not RNFL thinning (Fig. 2), but the difference was not significant (74.9 ± 6.3 pg/ml and 64.1 ± 5.2 pg/ml, respectively, p > 0.05).
Discussion The current study found that both the total study sample of patients with active uveitis complicated by optic nerve inflammation, and those with RNFL thinning, had significantly increased TNF-α and IL-1β levels compared to healthy controls. These findings are consistent with other reports on elevated serum TNF-α levels in such patients [8-11, 21, 22]. However, our findings differ from those of some studies [2, 5, 23] that have reported no significant difference in serum TNF-α levels between uveitis patients and healthy controls. Our finding of elevated TNF-α levels in RNFL thinning secondary to uveitis are indirectly confirmed by a study by Katome et al. (2013) [24], who found that the reduced production of microglial TNF-α after optic nerve injury results in the higher rate of retinal ganglion cell survival in apoptosis signal-regulating kinase 1 (ASK1) knockout mice. Another finding of the current study is significantly increased serum IL-1β levels in patients with uveitis complicated by optic nerve inflammation, with a 17% difference in serum IL-1β level between those who had and those who had not RNFL thinning secondary to uveitis. This is in line with findings of increased serum IL-1β levels in patients with Behçet's disease by Chekaoui et al [7] and Evereklioglu et al [9], but does not confirm reports of the absence of significant changes in serum IL-1β levels in patients with uveitis in studies by others [2, 4]. Our findings should be considered in conjunction with (1) those by Tseng et al [13] (2013) who found that IL-1β is involved in caspase-1-mediated cell death, known as "pyroptosis", and (2) those by Bariş et al [25] (2016) who reported that the IL-1β gene polymorphism was statistically correlated with the presence of an ocular lesion in patients with Behçet's disease. We also found that, in active stage of uveitis, (1) the serum TNF-α level was significantly, more than 1.5 times, higher in patients with RNFL thinning due to uveitis complicated by optic nerve inflammation compared to patients with normal RNFL thickness, and (2) the serum IL-1β level was higher in the former patients than in the latter patients, but the difference was not significant. This is in agreement with findings of Kitaoka et al [26] (2016) that IL-1β was upregulated in astrocytes in the optic nerve in TNF-induced optic nerve degeneration in rats, and with findings of Sivakumar et al [27] (2011) that excess production of TNF-α and IL-1β by microglia can induce retinal ganglion cell death in the retina of one-day-old Wistar rats subjected to hypoxia. Moreover, our findings of elevated serum TNF-α levels in patients with RNFL thinning are in indirect agreement with the results of our previous study [28] that concentration of matrix metalloproteinase-9 in uveitis complicated by optic nerve inflammation correlated with the development of partial optic nerve atrophy, since Yamada et al [29] showed in their experimental study that the expression of MMP-9 increased in the presence of TNF-α. Therefore, the current study demonstrated that, in active stage of uveitis, (1) the serum TNF-α level was significantly, more than 1.5 times, higher in patients with RNFL thinning due to uveitis complicated by optic nerve inflammation compared to patients with normal RNFL thickness, and (2) the serum IL-1β level was 17% higher in the former patients than in the latter patients, but the difference was not significant. The results of the study may justify the use of biologic therapy in the treatment of uveitis complicated by optic nerve inflammation.
References 1.Abu El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SA, Opdenakker G, Geboes K, Van Damme J. Cytokine and CXC chemokine expression patterns in aqueous humor of patients with presumed tuberculous uveitis. Cytokine. 2012;59(2):377–81. 2.Chen W, Zhao B, Jiang R, Zhang R, Wang Y, Wu H, Gordon L, Chen L. Cytokine expression profile in aqueous humor and sera of patients with acute anterior uveitis. Curr Mol Med. 2015;15(6):543-9. 3.Hernández Garfella ML, Palomares Fort P, Román Ivorra J, Cervera Taulet E. Aqueous Humor Levels of Different Interleukins 1-β, 2, 6 and 10, Tumor Necrosis Factor-α and Vascular Endothelial Growth Factor in Uveitis Treated with Adalimumab. J Ophthalmic Vis Res. 2015 Jan-Mar;10(1):49-54. 4.Zhao B, Chen W, Jiang R, Zhang R, Wang Y, Wang L, Gordon L, Chen L. Expression profile of IL-1 family cytokines in aqueous humor and sera of patients with HLA-B27 associated anterior uveitis and idiopathic anterior uveitis. Exp Eye Res. 2015;138:80-86. 5.Nagata K, Maruyama K, Uno K, Shinomiya K, Yoneda K, Hamuro J, Sugita S, Yoshimura T, Sonoda KH, Mochizuki M, Kinoshita S. Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Invest Ophtalmol Vis Sci. 2012;53(7):3827–33. 6.Shibata M, Sato T, Taguchi M, Tanaka A, Harimoto K, Takeuchi M. Analysis of Cytokines Related to Helper T and Regulatory T Cells in the Vitreous of Uveitis Patients. Nippon Ganka Gakkai Zasshi. 2015 Jun;119(6):395–401. 7.Chekaoui A, Lahmar K, Belguendouz H, Mazari F, Terahi M, Hakem D, Youinou P, Touil-Boukoffa C. Increased IL-1β levels are associated with an imbalance of "oxidant/antioxidant" status during Behçet's disease. Eur Cytokine Netw. 2018;29(3):95-102. 8.Cordero-Coma M, Calleja S, Llorente Rodriguez E, Franco M, Ruiz de Morales JG. Serum cytokine profile in adalimumab-treated refractory uveitis patients: decreased IL-22 correlates with clinical responses. Ocul Immunol Inflamm. 2013 Jun; 21(3):212–9. 9.Gholijani N, Ataollahi MR, Samiei A, Aflaki E, Shenavandeh S, Kamali-Sarvestani E. An elevated pro-inflammatory cytokines profile in Behcet's disease: A multiplex analysis. Immunol Lett. 2017;186:46-51. 10.Mesquida М. Molins B, Llorens V, Sainz de la Maza M, Hernandez MV, Espinosa G, Adán A. Proinflammatory cytokines and C-reactive protein in uveitis associated with Behcet's disease. Mediators Inflamm [Internet]. 2014 Jun 8. 11.Türkcü FM, Şahin A, Cingü AK, Kaya S, Yuksel H, Cinar Y, Batmaz I. Serum omentin, resistin and tumour necrosis factor-α levels in Behcet patients with and without ocular involvement. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1565–68. 12.Zhao R, Zhou H, Zhang J, Liu X, Su SB. Interleukin-1β promotes the induction of retinal autoimmune disease. Int Immunopharmacol. 2014;22(2):285-92. 13.Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D'Amore PA, Ksander BR. NLRP3 Inflammasome Activation in Retinal Pigment Epithelial Cells by Lysosomal Destabilization: Implications for Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2013;54(1):110-20. 14.Mac Nair CE, Fernandes KA, Schlamp CL, Libby RT, Nickells RW. Tumor necrosis factor alpha has an early protective effect on retinal ganglion cells after optic nerve crush. J Neuroinflammation. 2014;11:194. 15.Kozak de Y, Naud MC, Bellot J, Faure JP, Hicks D. Differential Tumor Necrosis Factor Expression by Resident Retinal Cells From Experimental Uveitis-Susceptible and -Resistant Rat Strains. J Neuroimmunol. 1994 Nov;55(1):1-9. 16.Kitaoka Y, Sase K, Tsukahara C, Kojima K, Shiono A, Kogo J, Tokuda N, Takagi H. Axonal protection by ripasudil, a Rho kinase inhibitor, via modulating autophagy in TNF-Induced optic nerve degeneration. Invest Ophthalmol Vis Sci. 2017;58:5056–64. 17.Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol Res. 1996;18(2):176-84. 18.Tsukahara C, Sase K, Fujita N, Takagi H, Kitaoka Y. Axonal Protection by Tacrolimus with Inhibition of NFATc1 in TNF-Induced Optic Nerve Degeneration. Neurochem Res. 2019;44(7):1726-35. 19.Paschalis EI, Zhou C, Lei F, Scott N, Kapoulea V, Robert MC, Vavvas D, Dana R, Chodosh J, Dohlman CH. Mechanisms of Retinal Damage after Ocular Alkali Burns. Am J Pathol. 2017;187(6):1327-42. 20.Panchenko NV, Samofalova MN, Gonchar EN, Prikhodko DO, Arustamova GS, Litvishenko AV. [Alterations in the expression of tumor necrosis factor-alpha in the uveitis with optic nerve inflammation]. Oftalmol Zh. 2016;5:18-21. Russian. 21.Evereklioglu C, Er H, Turkoz Y, Cekmen M. Evereklioglu C. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet's disease. Mediators Inflamm. 2002 Apr;11(2):87–93. 22.Santos Lacomba M, Marcos Martín C, Gallardo Galera GM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, Omar M. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. Sep-Oct 2001;33(5):251-5. 23.Takase H, Futagami Y, Yoshida T, Kamoi K, Sugita S, Imai Y, Mochizuki M. Cytokine Profile in Aqueous Humor and Sera of Patients with Infectious or Noninfectious Uveitis. Invest Ophtalmol Vis Sci. 2006;47(4):1557–61. 24.Katome T, Namekata K, Guo X, Semba K, Kittaka D, Kawamura K, Kimura A, Harada C, Ichijo H, Mitamura Y, Harada T. Inhibition of ASK1-p38 pathway prevents neural cell death following optic nerve injury. Cell Death Differ. 2013;20(2):270-80. 25.Bariş S, Akyürek Ö, Dursun A, Akyol M. The impact of the IL-1β, IL-1Ra, IL-2, IL-6 and IL-10 gene polymorphisms on the development of Behcet's disease and their association with the phenotype. Med Clin (Barc). 2016;146(9):379-83. 26.Kitaoka Y, Tanito M, Kojima K, Sase K, Kaidzu S, Munemasa Y, Takagi H, Ohira A, Yodoi J. Axonal protection by thioredoxin-1 with inhibition of interleukin-1β in TNF-induced optic nerve degeneration. Exp Eye Res. 2016;152:71-76. 27.Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C. Retinal Ganglion Cell Death Is Induced by Microglia Derived Pro-Inflammatory Cytokines in the Hypoxic Neonatal Retina. J Pathol. 2011;224(2):245-60. 28.Panchenko NV, Samofalova MN, Friantseva MV. [Concentration of matrix metalloproteinase-9 at different outcome of uveitis complicated by inflammation of the optic optic nerve]. Oftalmologiia. Vostochnaia Evropa. 2016;6(2):210-6. Russian. 29.Yamada H, Yoneda M, Inaguma S, Watanabe D, Banno S, Yoshikawa K, Mizutani K, Iwaki M, Zako M. Infliximab counteracts tumor necrosis factor-α-enhanced induction of matrix metalloproteinases that degrade claudin and occludin in non-pigmented ciliary epithelium. Biochem Pharmacol. 2013 Jun;85(12):1770–82. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|