J.ophthalmol.(Ukraine).2020;6:8-13.
|
http://doi.org/10.31288/oftalmolzh20206813 Received: 15 May 2020; Published on-line: 21 December 2020 Comparing the microflora of the conjunctiva and contact lens in therapeutic soft contact lens wearers T. A Veliksar., T. B. Gaidamaka, G. I. Drozhzhina, A. L. Molodaia, O. N. Ivanova, L. V. Dolenko, V. N. Konakh 1 SI "Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) E-mail: tveliksar@gmail.com TO CITE THIS ARTICLE: Veliksar TA, Gaidamaka TB, Drozhzhina GI, Molodaia AL, Ivanova ON, Dolenko LV, Konakh VN. Comparing the microflora of the conjunctiva and contact lens in therapeutic soft contact lens wearers. J.ophthalmol.(Ukraine).2020;6:8-13.http://doi.org/10.31288/oftalmolzh20206813
Background: More than 125 million (2%) people worldwide wear contact lenses (CL). Rates of CL-associated infections increase with an increase in frequency of wear of CL. Purpose: To compare the bacterial and fungal microflora of the conjunctiva and contact lens in therapeutic soft contact lens (SCL) wearers. Material and Methods: We examined the microflora of the conjunctival surface in 80 therapeutic SCL wearers and of the SCL surface in 235 relevant lenses. Monthly therapeutic SCLs that were discarded after being replaced were sent for bacteriological and mycological culture. We used only the data for the years 2016 through 2019 in the analysis. Results: Pathogenic and/or opportunistic agents were found in samples from 121 SCL (51.5%). Gram positive, Gram negative and fungal species were found in 63.5%, 29.5%, and 7.0%, respectively, of the 121 SCL. Mixed microflora was evident in 14.1% of the 121 SCL. Organisms were isolated from the conjunctiva of 51 (63.8%) of 80 patients. Gram positive, Gram negative and fungal species were found in 75.0%, 20.0%, and 5.0%, respectively, of patients. Mixed microflora was evident in 14.1% of patients. Conclusion: Organisms were isolated from the conjunctiva of 51 (63.8%) of 80 therapeutic SCL wearers and from 121 (51.5%) of 235 SCL (p = 0.06). Conjunctival swabs and SCL swabs harbored pathogenic microorganisms on 26.2% and 26.9% of occasions, respectively. On 32.5% of occasions, there was a difference in species isolated from conjunctival swabs and SCL swabs. This stresses the need for microbiological studies of the conjunctiva and contact lenses in SCL wearers. Keywords: microbiological studies, conjunctiva, soft contact lens, bacterial flora, fungal flora, antibacterial agents
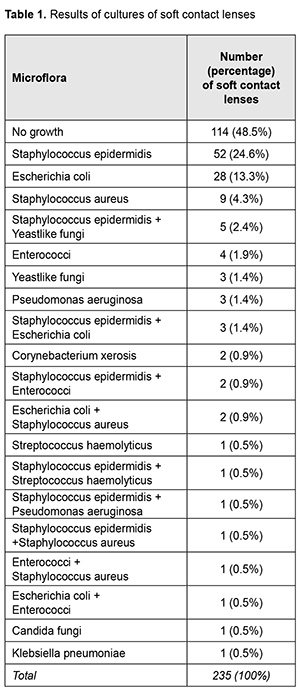
Introduction More than 125 million (2%) people worldwide wear contact lenses (CL) and 0.011 - 0.2% of the wearers develop keratitis per year [1]. Rates of CL-associated infections increase with an increase in frequency of wear of CL. Many studies have attempted to define the sources of bacteria that contaminate CL. To date, microorganisms that colonize lid margins [2], hands [3], lens cases [4], and the domestic water supply [2] have been implicated as such sources. In addition, associations of lens contamination with the physical properties of and lens care procedures for the usage and care of CL have been demonstrated [5, 6]. The normal ocular surface microflora includes coagulase-negative staphylococci, Corynebacterium, Micrococcus, Bacillus and Propionibacterium [7, 8]. The frequency of isolation of conjunctival microorganisms during soft lens wear varies between 15–90% in the literature [7, 9]. However, most studies report the conjunctiva and lid margin are sparsely populated with organisms at a frequency of about 30% and 70%, respectively [8]. Previous studies report conflicting results on the association between lens microbial contamination and conjunctival microflora [2]. It has been shown that during asymptomatic wear of low Dk soft lenses, the likely route for normal ocular microflora to colonize lenses is via the lid margins. A study by Szczotka-Flynn [10] found a greater diversity of bacteria on the silicone hydrogel study contact lenses (14 types) than either lids (11 types) or conjunctivae (7 types). On 257 occasions where lenses harbored organisms, 83.8% had the same species cultured from lids and lenses. Only 26.1% had the same species detected on lenses and conjunctival swabs [10]. During asymptomatic soft contact lens wear, some authors have reported an increase in ocular microflora with lens wear [7]. Specifically, an increase in numbers but not types of organisms were found on lid margins, or conjunctivae with soft contact lens wear, and more potential pathogens have been recovered from the conjunctivae of extended wear users compared to daily wear users. The frequency of positive conjunctival cultures (CNS and Corynebacterium species) rose by 56% after 30-day continuous wear [7,8]. Contact lens usage affects the balance between staphylococci and corynebacteria in conjunctival microflora and causes the advance of staphylococci [11]. Serratia spp., Staphylococcus aureus and coagulase-negative staphylococci were found to be the most common organisms isolated from contact lenses [6]. The most and the least contaminated samples were found to be lens cases (62%) and lens care solution (42%), respectively [12]. A lack of microbiological findings in around 51.2 % of CS made the researchers recommend increasing the microbiological analysis of CL and CL containers in order to improve current treatment strategies in future [1]. The purpose of the study was to compare the bacterial and fungal microflora of the conjunctiva and contact lenses in therapeutic SCL wearers. Material and Methods We examined the microflora of the conjunctival surface in 80 therapeutic SCL wearers and of the SCL surface in 235 lenses. Monthly therapeutic SCLs that were discarded after being replaced were subject to the study. We used only the data for the years 2016 through 2019 in the analysis. Microbiological (bacterial and mycological) studies were conducted at the certified microbiological laboratory of the Filatov institute (certificate No. PT-236/18). Blood agar containing 5% red blood cells to provide nutrients was used as a media for isolation of microbiological pathogens; sterility control medium was also used. Primary isolation plates were incubated at (37±1) °С for 24 h, and, if the growth was observed, colonies were picked up, and Gram staining and microscopy were performed. Depending on the morphology, selective media was used to allow the growth of certain organisms, and, subsequently, get pure cultures. If no growth was apparent after 24 h, the plates were incubated for another 24 h. Organism detection under aseptic conditions was considered as either evidence of involvement of the organism in ocular tissue inflammation or indicator of high risk for inflammation. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The parametric Student t test was used for unpaired samples. The level of significance p ≤ 0.05 was assumed. Results No microbiological growth was observed for samples from 114 SCL (48.5%) and pathogenic and/or opportunistic agents were found in samples from 121 SCL (51.5%). Gram positive, Gram negative and fungal species were found in 63.5%, 29.5%, and 7.0%, respectively, of SCL. Mixed microflora was evident in 14.1% of SCL, with the most common combination being Staphylococcus epidermidis and yeastlike fungi (Table 1).
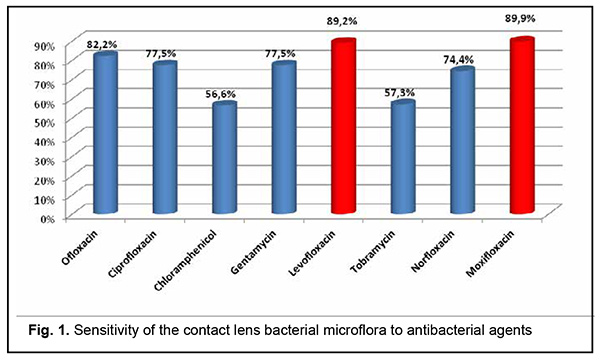
Among the identified pathogens, Staphylococcus epidermidis was the most common (47.1%, including 9.4% attributed to its combinations with pathogenic microflora), followed by Escherichia coli (24.6%), Staphylococcus aureus (9.4%), yeastlike fungi (5.8%), enterococci (5.8%), Pseudomonas aeruginosa (2.9%), Streptococcus haemolyticus (1.4%), Corynebacterium xerosis (1.4%), Candida fungi (0.7%), and Klebsiella pneumoniae (0.7%). The bacterial microflora cultured from SCL was most commonly sensitive to moxifloxacin (89.9%), followed by levofloxacin (82.9%); ofloxacin (82.9%), ciprofloxacin (77.5%); gentamycin (77.5%), norfloxacin (74.4%); tobramycin (57.3%), and chloramphenicol (56.6%) (Fig. 1).
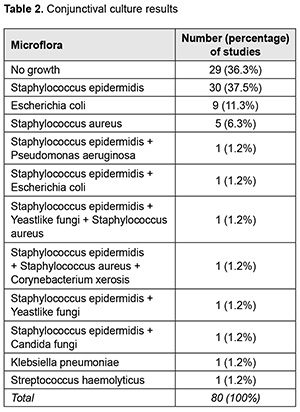
Organisms were isolated from the conjunctiva of 51 (63.8%) of 80 patients. Gram positive, Gram negative and fungal species were found in 75.0%, 20.0%, and 5.0%, respectively, of patients. Mixed microflora was evident in 14.1% of patients. Staphylococcus epidermidis was the most common (40.5%), followed by Escherichia coli (11.2%), Staphylococcus aureus (7.9%), yeastlike fungi (2.3%), Pseudomonas aeruginosa (1.1%), Candida fungi (1.1%), Klebsiella pneumoniae (1.1%), Streptococcus haemolyticus (1.1%), and Corynebacterium xerosis (1.1%) (Table 2).
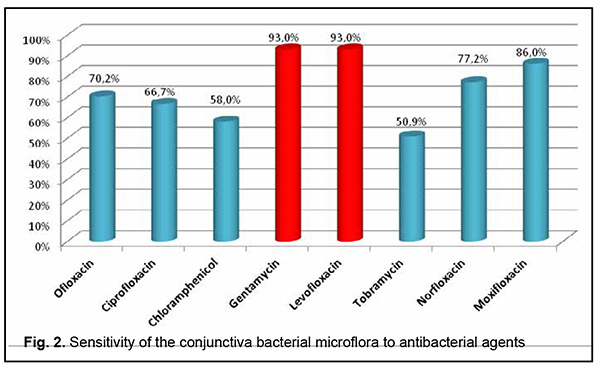
There was no significant difference (p = 0.06) in the frequency of detection of microflora or in composition of microflora between conjunctival swabs and SCL swabs. The bacterial microflora cultured from the conjunctiva was most commonly sensitive to gentamycin (93.0%) and levofloxacin (93.0%), followed by moxifloxacin (86.0%), norfloxacin (77.2%), ofloxacin (70.2%), ciprofloxacin (66.7%), chloramphenicol (58.0%) and tobramycin (50.9%) (Fig. 2).
Staphylococcus epidermidis cultured from the conjunctiva or SCL was most commonly sensitive to moxifloxacin (93.1%), followed by levofloxacin (88.1%), gentamycin (86.1%), norfloxacin (76.2%); ofloxacin (75.2%), ciprofloxacin (73.3%), tobramycin (61.4%) and chloramphenicol (60.4%) (Fig. 3).
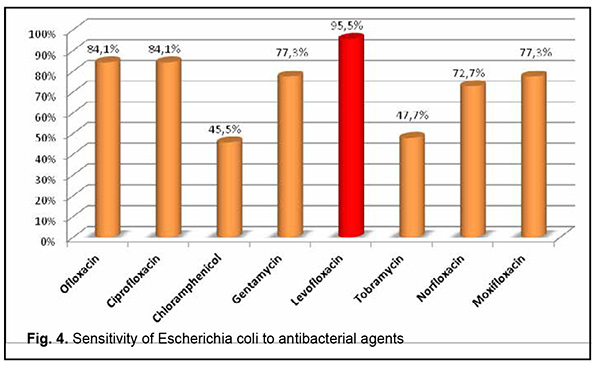
Escherichia coli cultured from the conjunctiva or SCL was most commonly sensitive to levofloxacin (95.5%), followed by ofloxacin (84.1%), ciprofloxacin (84.1%), gentamycin (77.3%), moxifloxacin (77.3%), norfloxacin (72.7%), tobramycin (47.7%) and chloramphenicol (45.5%) (Fig. 4).
Staphylococcus aureus was most commonly sensitive to moxifloxacin (95.0%), followed by gentamycin (90.0%), levofloxacin (90.0%), chloramphenicol (90.0%), norfloxacin (80.0%), ofloxacin (65.0%), ciprofloxacin (55.0%), and tobramycin (45.0%) (Fig. 5).
Pseudomonas aeruginosa was most commonly sensitive to ofloxacin (100.0%), ciprofloxacin (100.0%), and levofloxacin (100.0%), followed by norfloxacin (60.0%), moxifloxacin (60.0%), and gentamycin (40.0%), and not sensitive to tobramycin or chloramphenicol. Streptococcus haemolyticus was sensitive to ofloxacin, ciprofloxacin, gentamycin, levofloxacin, norfloxacin and moxifloxacin in all cases, and to chloramphenicol and tobramycin in 66.7% and 33.3%, respectively. Enterococcus was most commonly sensitive to moxifloxacin (87.5%), ciprofloxacin (87.5%), levofloxacin (87.5%), tobramycin (62.5%), gentamycin (50.0%), norfloxacin (50.0%), and chloramphenicol (50.0%). Yeastlike fungi were weakly sensitive to antifungal agents. They were most sensitive to itraconazol (30.0%) and clotrimazole (30.0%), followed by voriconazole (20.0%), ketoconazole (20.0%) and amphotericine B (20.0%), and not sensitive to nystatin. Candida fungi were sensitive to clotrimazole and nystatin, and resistant to itracon, amphotericine B, voriconazole and ketoconazole. On 32.5% of occasions, there was a difference in species isolated from conjunctival swabs and SCL swabs. Specifically, on 13.8% of occasions, there was no growth in the sample from SCL, but an organism (Staphylococcus epidermidis, 63.6%; Escherichia coli, 9.1%; Candida fungi, 9.1%; Staphylococcus aureus, 9,1%; and enterococci, 9,1%) was isolated from the conjunctiva. In addition, on 10.0% of occasions, there was no growth in the sample from the conjunctiva, but an organism (Staphylococcus epidermidis, 37.5%; Escherichia coli, 37.5%; and Staphylococcus aureus, 12.5%) was isolated from the SCL. We noted that, with Staphylococcus epidermidis isolated from the conjunctiva, pathogenic organisms were isolated from 5.1% of the relevant SCL (Escherichia coli, 2.5%; Pseudomonas aeruginosa, 1.3%; and yeastlike fungi, 1.3%). In addition, with Staphylococcus epidermidis isolated from the SCL, yeastlike fungi were isolated from 1.3% of the relevant conjunctival samples. Discussion According to Szczotka-Flynn L. B., only 26.1% had the same species detected on lenses and conjunctival swabs [9]. In our study, the same species were noted in 67.5% cases. Conjunctival swabs and SCL swabs harbored pathogenic microorganisms (Escherichia coli, Staphylococcus aureus, Streptococcus haemolyticus, Pseudomonas aeruginosa, yeastlike fungi and Candida fungi) on 26.2% and 26.9% of occasions, respectively, with Escherichia coli, Staphylococcus aureus being the two most commonly isolated pathogenic organisms. Our data were consistent with those of Iskeleli G. [4] demonstrating that there was no difference in organisms isolated from the conjunctiva and SCL in symptom-free SCL wearers. However, we detected no difference in the frequency of microflora. Based on our data and similar to Böhm M. R. R. [1], we recommend performing the microbiological analysis of both the conjunctiva and SCL in order to improve diagnosis, treatment strategies and outcomes. Since, on 32.5% of occasions, there was a difference in species isolated from conjunctival swabs and SCL swabs, we recommend microbiological studies of the conjunctiva and contact lenses in SCL wearers in order to avoid infectious complications and assign а proper treatment. Levofloxacin, moxifloxacin and gentamycin were found to be the most effective, whereas chloramphenicol and tobramycin were found to be the least effective antibacterial agents in SCL wearers. References 1.Böhm MR, Prokosch V, Merté RL, Koch R, Busse H, Stupp T. Böhm MR, et al. [Microbiological Analysis in Contact Lens-Associated Keratits]. Klin Monatsbl Augenheilkd. 228(9):808–14. German. 2.Willcox MD, Power KN, Stapleton F, et al. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997 Dec;74(12):1030-8. 3.Mowrey-McKee MF, Sampson HJ, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part II: Quantitation of microbes after patient handling and after aseptic removal from the eye. CLAO J. 1992 Oct;18(4):240-4. 4.Boost MV, Cho P. Microbial flora of tears of orthokeratology patients, and microbial contamination of contact lenses and contact lens accessories. Optom Vis Sci. 2005 Jun;82(6):451-8. 5.Iskeleli G, Bahar H, Unal M, et al. Microbiologic evaluation of frequent-replacement soft contact lenses. CLAO J. 2002 Oct;28(4):192-5. doi: 10.1097/01.ICL.0000024118.45191.9B. 6.Yung MS, Boost M, Cho P, Yap M. Microbial contamination of contact lenses and lens care accessories of soft contact lens wearers (university students) in Hong Kong. Ophthalmic Physiol Opt. 2007 Jan;27(1):11-21. 7.Iskeleli G, Bahar H, Eroglu E, Torun MM, Ozkan S. Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens. 2005 May;31(3):124-6.f. 8.Stapleton F, Willcox MD, Fleming CM, et al. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun. 1995 Nov;63(11):4501-5. 9.Fleiszig SM, Efron N. Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol. 1992 May; 30(5): 1156–61. 10.Szczotka-Flynn LB, Bajaksouzian S, Jacobs MR, Rimm A. Risk Factors for Contact Lens Bacterial Contamination During Continuous Wear. Optom Vis Sci. 2009 Nov;86(11):1216-26. 11.Kozer-Bilgin L, Demir N, Altan-Yaycioglu R. Microbiological evaluation of contact lenses and contact lens disinfection solutions in an asymptomatic population and in medical personnel. CLAO J. 1999 Oct;25(4):228-32. 12.Thakue DV, Gaikwad UN. Microbial Contamination of Soft Contact Lenses & Accessories in Asymptomatic Contact Lens Users. Indian J Med Res. 2014 Aug;140(2):307-9. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|