J.ophthalmol.(Ukraine).2020;5:36-42.
|
http://doi.org/10.31288/oftalmolzh202053642 Received: 27 July 2020; Published on-line: 27 October 2020 A novel concept of differences in pathogenetic mechanism of diabetic retinopathy progression between type 2 diabetes mellitus patients differing in the PPARγ genotype L. V. Natrus1, S. Yu. Mogilevskyy2, T. I. Panova1, S. O. Rykov2, M. Iu. Bykhovets2 1 Bohomolets National Medical University; 2 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) E-mail: Lnatrus777@gmail.com TO CITE THIS ARTICLE: Natrus LV, Mogilevskyy SYu, Panova TI, Rykov SO, Bykhovets MIu. A novel concept of differences in pathogenetic mechanism of diabetic retinopathy progression between type 2 diabetes mellitus patients differing in the PPARγ genotype. J.ophthalmol.(Ukraine).2020;5:36-42. http://doi.org/10.31288/oftalmolzh202053642 Background: It is important to study cellular circulation of L-FABP-associated fatty acids (FA) and their oxidation through the classical pathway with activation of the PPARγ gene and under conditions of impaired lipid metabolism. Purpose: To investigate the pathogenetic mechanisms of diabetic retinopathy (DR) progression in type 2 diabetes mellitus (T2DM) patients differing in the PPARγ genotype. Material and Methods: This study involved 101 T2DM patients (101 eyes) with different stages of diabetic retinopathy (DR) as assessed by the ETDRS scale and 40 non-diabetics (controls) who were comparable in age, gender and body mass index. The polymorphism was detected by real-time PCR on a Real-Time Gene Amp® PCR System 7500 (Applied Biosystems). ELISA was used to determine serum L-FABP levels with Human L-FABP ELISA kit (Hycult Biotech). Results: Our findings allowed for developing a concept of differences in pathogenetic mechanisms of DR progression between patients differing in the PPARγ genotype. Among carriers of the wild-type PPARγ genotype, DR as a complication of diabetes develops as a result of chronic inflammation and through PPARγ-dependent gene transcription, expression of the enzymes that oxidize arachidonic acid, and synthesis of the metabolites affecting the endothelium, platelets, the blood clotting system, etc. In diabetic PPARγ polymorphism carriers, PPARγ-dependent gene transcription is inhibited, and fatty acids are utilized in the cell via other L-FABP mechanisms, which results in activation of direct peroxisomal oxidation and increased oxidative stress-induced inflammation. A new insight into differences in pathogenetic mechanisms of DR progression between T2DM patients differing in the PPARγ genotype provides the basis for subsequent clinical development of advanced, customized management schemes for patients with different DR stages to prevent further retinal damage. Keywords: diabetic retinopathy, L-FABP, polymorphism
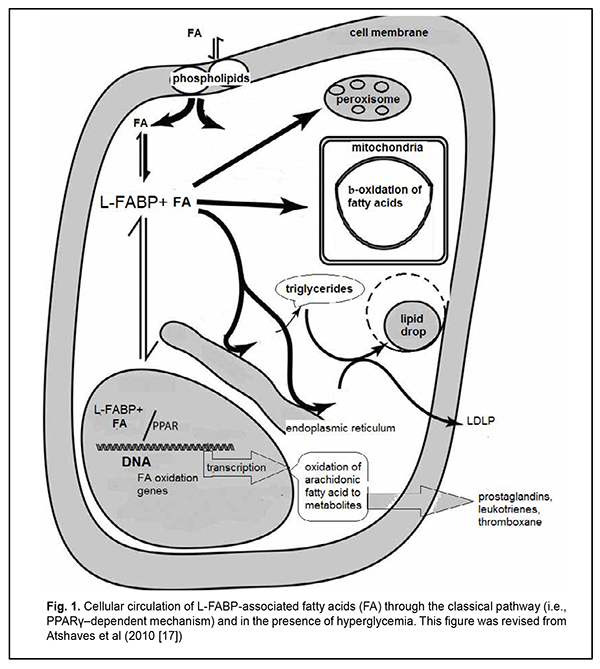
Introduction Diabetic retinopathy (DR) is the most common complication of diabetes mellitus and a leading cause of visual impairment and blindness [1-3]. Most patients with retinal diabetic lesions are those with type 2 diabetes mellitus (T2DM) [4]. Although the etiology and pathophysiology of DR have been studied over half a century, there is still a lack of effective treatment patterns aimed at controlling the pathologic condition and preventing disease progression and worsening. Vascular endothelium dysfunction in the presence of hypercholesteremia is considered an important factor in retinal damage due to lipotoxicity and chemical modification of vascular proteins. Vascular wall lipid peroxidation results in local production of reactive oxygen species (free radicals) that mediate macrophage recruitment and cell activation and proliferation. Therefore, hyperlipidemia may contribute to DR and macular edema by endothelial dysfunction and breakdown of the blood retinal barrier [5,6]. At the same time, there have been contradictory reports on the effect of lipid profile on retinopathy or maculopathy. Cetin and colleagues [7] found a significant correlation between HbA1c and total cholesterol, but there was no association between serum lipids and DR. The Multi-ethnic Study of Atherosclerosis (MESA) showed no association between DR and serum lipids, course of diabetes, obesity and lifestyle [6]. On the contrary, in a study by Rema and colleagues (the Chennai Urban Rural Epidemiology Study (CURES) Eye Study), the mean serum cholesterol, serum triglycerides and low-density lipoprotein cholesterol concentrations were higher in subjects with DR compared with those without DR. However, after adjusting for HbA(1c) and body mass index, only triglycerides maintained a significant association with DR [8]. Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors and belong to a nuclear hormone receptor superfamily. PPARγ has gained interest as a therapeutic target in the diseases in which dyslipidemia and insulin resistance are pathogenetically involved, such as metabolic syndrome, insulin resistance (IR), T2DM, obesity, and etc [9-11]. PPAR-γ physically interacts with fatty acid binding proteins (FABPs), the intracellular lipid chaperones, especially with liver FABP (L-FABP). It has been suggested that L-FABP could be considered a co-activator in PPAR-mediated gene regulation. As lipid chaperones, FABPs protect cells from excess fatty acid flux [12], and may actively facilitate the transport of lipids to specific compartments in the cell, such as to the lipid droplet for storage; to the endoplasmic reticulum for signaling, trafficking and membrane synthesis; to the mitochondria or peroxisome for oxidation; to the nucleus for lipid-mediated transcriptional regulation; or even outside the cell to signal in an autocrine or paractine manner [13]. The ability of FABPs to modulate lipid signalling and trafficking could be exploited for developing medications, improving therapeutic or prophylactic measures for adequate control of metabolic disorders, and controlling the activity of nuclear hormone receptors as therapeutic targets [9, 11]. However, the association between L-FABP expression and the PPARγ genotype in patients with DR, and the mechanisms implemented on the basis of above regulation components, remain unclear. The purpose of the study was to investigate the pathogenetic mechanisms of diabetic retinopathy progression in T2DM patients differing in the PPARγ genotype. Material and Methods This study involved 101 T2DM patients (101 eyes) from the Ukrainian population. They underwent an eye examination including visual acuity assessment, refractometry, static Humphrey perimetry, tonometry, and biomicroscopy. In addition, gonioscopy, ophthalmoscopy with a Goldmann contact lens, and macular optical coherence tomography (Topcon DRI OCT Triton, Tokyo, Japan) were performed, when required. Fundus photography (the ETDRS seven standard fields) was performed with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). Fluorescein angiography was performed when indicated. Diabetic retinopathy was graded using ETDRS scale. The patients were divided into three groups based on the stage of DR: group DR1 included 30 patients (30 eyes) with mild, moderate or severe non-proliferative DR (NPDR), group DR2 comprised 34 patients (34 eyes) with mild, moderate or severe proliferative DR (PDR), and group DR3 comprised 37 patients (37 eyes) with progressive proliferative PDR. The control group comprised 40 non-diabetics. All biochemical, molecular genetic and gas-liquid chromatography studies were performed at the certified laboratories of the Bohomolets NMU Research Institute for Experimental and Clinical Medicine [14-16]. ELISA was used to determine serum L-FABP levels with Human L-FABP ELISA kit (Hycult Biotech, Uben, the Netherlands). Gas-liquid chromatography was used to determine fatty acid composition of red blood cell (RBC) membranes following extraction of RBC mass from venous blood. The PPARG Pro12Ala SNP (rs1801282) was detected by real-time polymerase chain reaction (PCR). Mutation detection was performed using TaqMan Mutation Detection Assays (Thermo Fisher Scientific). The C allele is a considered a wild allele, whereas the G allele is considered a minor allele according to MAF Source: 1000 Genomes (http://www.1000genomes.org/node/506). Data were analyzed using IBM SPSS version 23 and MedStat software. Univariate analysis (Kruskal-Wallis test) was carried out for comparisons between groups. Median values were used for descriptive purposes. The level of significance p < 0.05 was assumed. Results Previously [16], we have described in detail the distribution of allele and genotype frequencies for PPARγ gene among groups of patients with different stages of DR, and showed that, compared to the controls, the percentage of carriers of the PPARG gene polymorphism was decreased in all DR groups. In addition, we have found substantial differences in concentrations of fatty acids in cell membranes, and L-FABP between patients with different PPARG gene-mediated phenotypes and different DR stages. More detailed analysis demonstrated that serum L-FABP level in the carriers of the Pro12Pro (wild) genotype of the control group was 9.77 ng/ml; DR1 group, 11.05 ng/ml; DR2 group, 15.2 ng/ml; and DR3 group, 16.76 ng/ml. Therefore, with an increase in DR stage, serum L-FABP level in the carriers of the Pro12Pro genotype increased 1.13-fold, 1.5-fold and 1.7-fold, respectively. In addition, serum L-FABP level in the carriers of the 12Ala allele of the DR1 group was 6.48 ng/ml; DR2 group, 26.13 ng/ml; and DR3 group, 10.81 ng/ml. This represents a 4-fold (р < 0.05), 2.36-fold, and 1.7-fold increase, respectively, compared to the control group. Because arachidonic acid (C20:4) is derived from its metabolic precursor, linoleic acid (C18:2), and this transformation is an important biological process, comparing the C20:4 to C18:2 ratio is essential for lipid metabolism analysis. Arachidonic acid metabolites are numerous biologically active compounds such as eicosanoids, prostaglandins and leukotrienes, endogenous ligands of cannabinoid receptors which are important intercellular regulators, but if produced in excess can promote a number of chronic disorders. Among the carriers of the Pro12Pro genotype, arachidonic acid level was significantly increased, especially in the DR1 group, and gradually decreased with an increase in severity of DR. The C20:4 to C18:2 ratio in the DR1 group was increased 2.2-fold (р<0.05), and in the DR2 group, 1.25-fold, compared to the control group. In addition, in the DR3 group, the ratio was too small and more than 3 times smaller compared to the control group, which reflects a low arachidonic acid level for this group. However, it is this condition (progressive proliferative PDR) that is associated with long-standing chronic inflammation and development of endothelial dysfunction. Among the carriers of the 12Ala allele, the C20:4 to C18:2 ratio in the DR1 group was increased 1.3-fold (р<0.05), and in the DR2 group, 1.2-fold, whereas in the DR3 group, decreased 2.5-fold, compared to the control group. Discussion Therefore, according to findings from recent studies, the classical pathway for the fatty acids entering the cytosol is implemented via their binding to L-FABP (Fig. 1).
Protein expression in the cell depends on the total concentration of fatty acids entering the cytosol, to make the incoming energy substrate in the form of fatty acids distributed over several sites [17]. Peng and colleagues [18] showed that serum TG levels were positively associated with serum logLFABP. However, the main signal of the presence of fatty acids, their concentrations and requirement for their utilization inside the cell gets to the nucleus to initiate genes transcription at relevant DNA sites with subsequent intracellular synthesis of the proteins required for this utilization and total fatty acid metabolism. Normally, the body is supplied with the amount of fatty acids required to maintain balanced lipid metabolism, with energy expenditure taken into account. Increased arachidonic acid oxidation with decreased arachidonic acid level in the cell and consequently increased plasma levels of arachidonic acid metabolites (prostaglandins, leukotrienes, etc.), inflammation markers, are considered a sign of cellular lipid overload [17,18]. We have previously reported that the main feature distinguishing the lipid metabolism in T2DM patients from that in non-diabetics was (1) a significant variation in fatty acid levels in red blood cell membranes as well as (2) elevated serum L-FABP level. Non-diabetics with increased blood glucose and cholesterol levels showed a decreased body mass index and insignificantly increased polyunsaturated FA levels in red blood cell membranes compared to relatively healthy individuals. In addition, serum L-FABP level in these non-diabetics was 1.2-fold decreased compared to relatively healthy individuals (p > 0.05) and 2-fold decreased compared to T2DM patients (p < 0.05) [14]. This suggests that under certain conditions, in the presence of metabolic shifts, it is a decreased L-FABP expression with increased polyunsaturated FA levels in cells that may prevent the development of obesity and diabetes. L-FABP level in patients with early DR and long duration of T2DM was 1.5-fold higher (p < 0.05) compared to the control group. In addition, L-FABP level increased 1.5-fold (р < 0.05), 1.3-fold, and 1.7-fold in the DR1, DR2 and DR3 groups, respectively, compared to controls. We believe that a reduced L-FABP expression in the DR2 group compared to the DR1 group reflects the body’s adaptation to high levels of plasma fatty acids, and has a hampering effect on DR progression [15]. Our findings suggest that fatty acid delivery to the cell may increase if a certain metabolic shift (e.g., hyperglycemia) occurs. However, L-FABP expression decreases, acting as a compensatory mechanism. Inflammation marker protein production as well as the classical pathway by which PPAR regulate gene transcription becomes suppressed, but metabolism of residual fatty acids in other organelles of the cell takes place. Insignificantly increased serum levels of triglycerides, total cholesterol and its subfractions (especially LDL cholesterol) are a marker of these changes [18]. A decrease in L-FABP level thus plays a protective role, preventing inflammation. Therefore, the above constitutes the basis for supplementing a traditional concept of low-grade, chronic, systemic inflammation in the presence of IR and T2DM which contributes to endothelial damage and causes microvascular complications, with new considerations of a significant difference in implementation of pathogenetic components between patients differing in PPARγ genotype (Figs. 2, 3). As we mentioned above, a decrease in L-FABP may play a protective role and suppress inflammation in a compensatory way. Under conditions of further development of insulin resistance, with a cell lacking glucose for a long time, the homeostasis regulation system changes the energy delivery pathway to beta oxidation, and the conditions develop for a typical increased fatty acid delivery to the cell. A diet rich in saturated fatty acids (especially, palmitic acid, a stimulator of L-FABP expression) significantly increases the expression of L-FABP, but the protein immediately forms a complex with fatty acids (the L-FABP/fatty acid complex). The signal entering the nucleus activates natural PPARγ-dependent gene transcription, and the cell begins actively oxidizing fatty acids (Fig. 1).
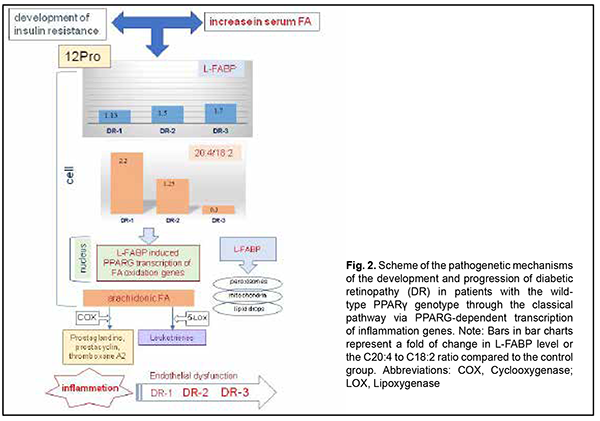
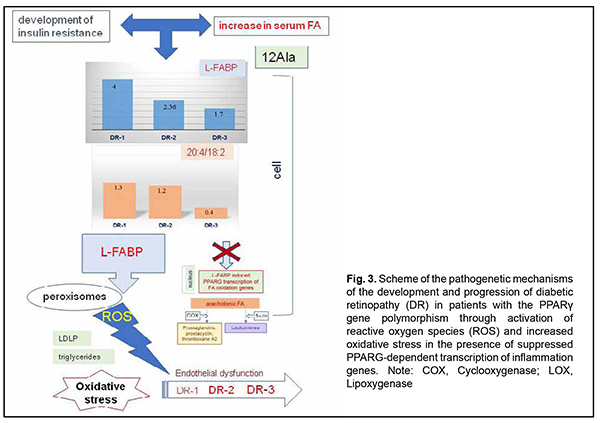
Increased C20:4 to C18:2 ratio and increased arachidonic acid concentration which we found in the DR1 group (Fig. 2) lead to activation of the cyclooxygenase or lipoxygenase pathway, arachidonic acid oxidation and increased plasma levels of prostaglandins, As the grade of retinal damage increases with time to that found in patients of the DR2 and DR3 groups, the level of arachidonic acid in the cell membrane decreases, and inflammation becomes chronic and more severe. The effect of arachidonic acid metabolites on the state of the endothelium has been well described and it was demonstrated that vasoconstriction, increased platelet aggregation, and capillary wall impairment participate in a microcirculatory alteration [5,20,21]. Therefore, the L-FABP level is inversely associated with the arachidonic acid level, because, as we suppose, the protein is actively utilized through L-FABP/fatty acid complexes that activate transcription processes in the nucleus. The cell converts the C20:4 substrate into metabolites, and, as the amount of the substrate decreases, the amount of accumulated protein increases. We believe that the above mechanism is actively implemented in carriers of the wild-type genotype of PPARγ. Another scenario is typical for PPARγ polymorphism carriers (Fig. 3). The degree of transcription of respective genes in response to stimulation by the L-FABP/fatty acid complex undergoes a change due to mutation, and arachidonic acid oxidation rate is substantially reduced Thus, we found that, compared with carriers of the wild-type genotype, PPARγ polymorphism carriers exhibited decreased arachidonic acid levels both in the control group and in the groups of patients with DR. The was practically no difference in the C20:4/C18:2 ratio between DR1, DR2 and control groups. At the same time, increased fatty acid levels entering the cell cause an increased L-FABP level with protein accumulation in the cell in PPARγ polymorphism carriers, and, at the DR1 stage, L-FABP expression was four-fold increased compared to carriers of the Pro12Pro genotype. Because the PPARγ-dependent mechanism of fatty acid utilization is inhibited by gene mutation, L-FABP does not form L-FABP/fatty acid complexes, but activates fatty acid oxidation at other sites of the cell. Thus, we have previously [15] found that, compared with carriers of the wild-type genotype, triglycerides and non-HDL-cholesterol concentrations in PPARγ polymorphism carriers were increased in patients with DR [19], which is in agreement with a study by Wang and colleagues [19]. In addition, it has been reported on increased peroxisomal oxidation and increased oxidative stress (OS) markers (malonic dialdehyde and diene conjugates) [22] and fatty acid accumulation in lipid droplets [16, 23] for 12Ala allele carriers. Others have reported that oxidative stress is a mechanism of early damage to the endothelial cell [5]. A study by Odegaard and colleagues [24] confirmed the correlation of OS biomarkers (F2-isoprostanes and oxidized LDL) with T2DM. Under conditions of glucolipotoxicity, OS causes free-radical lipid peroxidation [25] with accumulation of primary and secondary peroxidative breakdown products (diene conjugates and malonic dialdehyde and others, respectively) of phospholipids. Our findings and those in the literature suggest that, at the level of intracellular mechanisms, endothelial dysfunction in type 2 diabetics with retinopathy develops and increases through different pathways depending on the PPARγ genotype. Among carriers of the wild-type PPARγ genotype, DR as a complication of diabetes develops as a result of chronic inflammation and through PPARγ-dependent gene transcription, expression of the enzymes that oxidize arachidonic acid, and synthesis of the metabolites affecting the endothelium, platelets, and the blood clotting system. In diabetic PPARγ polymorphism carriers, PPARγ-dependent gene transcription is inhibited, and fatty acids are utilized in the cell via other L-FABP mechanisms, which results in activation of direct peroxisomal oxidation and increased oxidative stress-induced inflammation. The notion on the mechanisms of the development of DR in T2DM patients differing in the PPARγ genotype substantially improves our understanding of the pathogenesis of microcirculatory complications developing in the presence of hyperglycemia and dyslipidemia with involvement of L-FABP, a regulatory chaperone that, when involved in the complex with fatty acids, activates PPARγ-dependent gene transcription for utilization of cellular fatty acid lipids. Chronic inflammation and OS have been known to be important causes for microcirculatory complications of T2DM. However, our findings of differences in the degree of influence of the above-mentioned pathogenetic mechanisms, the sequence of complications and severity of late complications between T2DM patients differing in the PPARγ genotype provide the basis for developing advanced, customized management schemes for patients with different DR stages to prevent further retinal damage. We believe that the above-mentioned factors may influence also patients’ behavior (diet and physical activity habits and water intake schedule) and adaptation to therapeutic measures. These features should be taken into account when developing effective treatment schemes not only for groups differing in the stage of DR and duration of T2DM, but also for patients that differ in the polymorphisms of the genes of key lipid and carbohydrate metabolic enzymes. Therefore, the presence of behavioral differences between individuals differing in the PPARγ-dependent phenotype may be explained by genetically determined features of metabolic regulation, diet habits, and differences in regulatory effects on adipose tissue and mechanisms for supplying the liver and skeletal muscles with energy substrate. Research on patients’ phenotypic features that are based on the genetically determined mechanisms may be an important precondition for developing therapeutic schemes and nutritional and behavioral recommendations aimed at the prevention of DR progression in the presence of T2DM. Therefore, our findings allowed for developing a concept of differences in pathogenetic mechanisms of DR progression between patients differing in the PPARγ genotype, which is promising and provides the basis for subsequent clinical development of advanced, customized management schemes for patients with different DR stages to prevent further retinal damage. Acknowledgement: This study was a component of the research commissioned by the Ministry of Health of Ukraine (State Reg. No. 0119U101219) and planned for the years 2019-2021. References 1.Balashevich LI, Izmailov AS. [Diabetic ophthalmopathy]. St. Petersburg: Chelovek; 2012. Russian. 2.Shilova OG. [New aspects of the pathogenesis and treatment of diabetic retinopathy]. International journal of endocrinology. 2012;4(44). Russian. 3.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36. 4.Mogilevskyy SIu , Bushuieva OV, Natrus OV. [Features of diabetic retinopathy in patients with diabetes mellitus 2 type]. Arkhiv Oftal'mologii Ukrainy. 2017;5(1):37-44. Ukrainian. DOI: https://doi.org/10.22141/2309-8147.5.1.2017.172575. 5.Chernobryvtsev O.P. The endothelial dysfunction factors in diabetes mellitus 2 type. F J Educ Health Sport. 2019; 9 (1) : 410-416; 10.5281/zenodo.2608956. 6.Benarous R, Sasongko MB, Qureshi S, et al. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52(10):7464-9. 7.Cetin EN, Bulgu Y, Ozdemir S, et al. Association of serum lipid levels with diabetic retinopathy. Int J Ophthalmol. 2013;6(3):346-9. 8.Rema M, Srivastava BK, Anitha B, et al. Association of serum lipids with diabetic retinopathy in urban South Indians – the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-2. Diabet Med. 2006; 23(9):1029-36. 9.Grimaldi PA. Peroxisome proliferator-activated receptors as sensors of fatty acids and derivatives. Cell Mol Life Sci. 2007 Oct;64(19-20):2459-64. 10.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl M-C, et al. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000 Sep;26(1):76-80. 11.Petr M, Stastny P, Zajac A, et al. The Role of Peroxisome Proliferator-Activated Receptors and Their Transcriptional Coactivators Gene Variations in Human Trainability: A Systematic Review. Int J Mol Sci. 2018 May 15;19(5):1472. 12.Esteves A, Ehrlich R. Invertebrate intracellular fatty acid binding proteins. Comp Biochem Physiol C Toxicol Pharmacol. Mar-Apr 2006;142(3-4):262-74. 13.Choromańska B, Myśliwiec P, Dadan J, Hady HR, Chabowski A. The clinical significance of fatty acid binding proteins. Postepy Hig Med Dosw (Online). 2011 Nov 24;65:759-63. 14.Natrus LV, Gayova LV, Byjhovets MYu, Osadchuk YuS, Konovalov SE. [The value of regulatory effects on lipid metabolism in complicated type 2 diabetes mellitus]. Phyziologichnyi zhurnal. 2020;66(1):25-34. Ukrainian. 15.Rykov SO, Bykhovets MIu, Natrus LV. [Influence of L-FABP expression and fatty-acid composition of food on the lipid metabolism in patients with different degree of diabetic retinopathy and diabetes mellitus type 2]. Arkhiv Oftal'mologii Ukrainy. 2019; 7(3):27-36. Ukrainian. 16.Rykov SO, Natrus LV, Bykhovets MIu. PPARγ-mediated differences in energy substrate among T2DM patients differing in the stage of diabetic retinopathy. J Оphthalmol (Ukraine). 2019;6:7-14. 17.Atshaves BP, Martin G, Hostetler HA, et al. Liver Fatty Acid Binding Protein and Obesity. J Nutr Biochem. 2010 Nov;21(11):1015-32. 18.Peng X, Wu Y, Zhu Y, Huang R. Association of a Human FABP1 Gene Promoter Region Polymorphism with Altered Serum Triglyceride Levels. PLoS ONE. 2015 Oct 6;10(10):e0139417. 19.Wang Gu Qi, Bonkovsky HL, de Lemos A, Burczynski FJ. Recent insights into the biological functions of liver Fatty Acid Binding Protein 1. J Lipid Res. 2015 Dec;56(12):2238-47. 20.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002 Feb 5;105(5):546-9. 21.Mogilevskyy SYu, Panchenko YuO, Ziablitsev SV, Natrus LV. [Disturbed platelet aggregation as a factor of diabetic maculopathy and diabetic macular edema in patients with nonproliferative diabetic retinopathy in type 2 diabetes mellitus]. Arkhiv Oftal'mologii Ukrainy. 2018; 6(3):26-31. Ukrainian. 22.Mokrii YaV, Ziablitsev SV. [Effect of Pro12Ala polymorphism of PPARG gene on lipid peroxidation and antioxidant defense in patients with type 2 diabetes depending on the duration of the disease]. Patologiia. 2016;2(37):52-7. 23.Shi J, Zhang Y, Gu W, Cui B, Xu M, Yan Q, et al. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PLoS ONE. 2012;7(11): e48777. 24.Odegaard AO, Jacobs DR, Sanchez OA, et al. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016 Mar 24;15:51. 25.Su Y, Liu XM, Sun YM, Jin HB, Fu R, Wang YY, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008 Jun;62(6):877-82.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|