J.ophthalmol.(Ukraine).2020;5:29-35.
|
http://doi.org/10.31288/oftalmolzh202052935 Received: 17 February 2020; Published on-line: 27 October 2020
Efficacy of adjunctive autologous serum in the comprehensive treatment of bacterial corneal ulcers in patients with diabetic mellitus O. V. Zavoloka, P. A. Bezditko Kharkiv National Medical University, Department of Ophthalmology; Kharkiv (Ukraine) E-mail: оlesya_zavoloka@yahoo.com TO CITE THIS ARTICLE: Zavoloka OV, Bezditko PA. Efficacy of adjunctive autologous serum in the comprehensive treatment of bacterial corneal ulcers in patients with diabetic mellitus. J.ophthalmol.(Ukraine).2020;5:29-35. http://doi.org/10.31288/oftalmolzh202052935 Background: The presence of diabetic keratopathy associated with diabetic corneal neuropathy should be taken into account in the management of corneal inflammation in patients with diabetes mellitus. Purpose: To determine the impact of adjunctive topical autologous serum eye drops on delayed healing of bacterial corneal ulcers in patients with type 1 diabetes mellitus (DM1). Material and Methods: We retrospectively reviewed the results of observing 19 DM1 patients (19 eyes) with bacterial corneal ulcers who exhibited delayed healing of corneal ulceration and whose corneal smears and scrapings taken one week after initiation of treatment were bacteriologically negative. Patients had a routine eye examination (visual acuity, tonometry, and slit-lamp biomicroscopy of anterior and posterior eye segments), bacteriological studies, fluorescein dye test, anterior eye OCT and non-contact corneal esthesiometry. Patients of the control group (9 individuals) continued receiving only background therapy (topical antiseptics, antioxidants, repairing agents, artificial tears, and mydriatics and systemic anti-inflammatory agents), whereas patients of the main group began receiving autologous serum eye drops in addition to background therapy of topical antiseptics, mydriatics and systemic anti-inflammatory agents. Clinical picture data for day 1 (visit 1, before administering the treatment) and days 7 (when corneal samples and scrapings were re-taken), 14 (when bacteriology results became available and main group patients were additionally administered autologous serum), 17, 21 and 24 were analyzed. Results: In DM1 patients with delayed healing of bacterial corneal ulcers, the use of adjunctive autologous serum eye drops after eradication of the offending microorganisms resulted in reductions in precorneal injection and size and depth of the ulcer defect and complete resolution of corneal infiltration and edema of the corneal tissue surrounding the ulcer as early as day 3, and recovery of all patients and a 32.6% improvement in corneal sensitivity on day 10. Conclusion: Using adjunctive topical autologous serum eye drops after eradication of the offending microorganisms was found to be efficacious in the comprehensive treatment of delayed healing of bacterial corneal ulcers in patients with DM1. Keywords: diabetes mellitus, bacterial corneal ulcer, autologous serum, corneal sensitivity
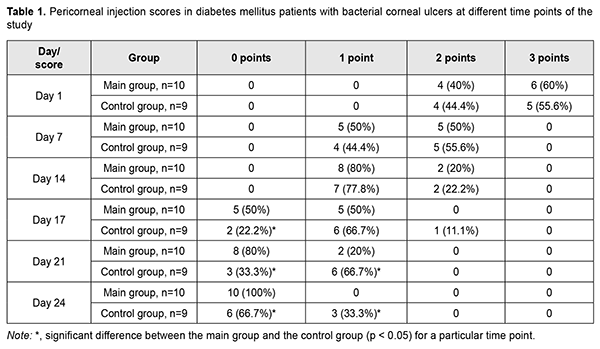
Introduction Management of corneal inflammation in patients with diabetes mellitus (DM) is a challenge for ophthalmic clinicians, since the latter should take into account a range of corneal structural and functional alterations that are typical for DM. Delayed corneal wound healing in patients with DM is caused by diabetic keratopathy developing in the presence of diabetic corneal neuropathy [1]. Reported prevalence of diabetic keratopathy in patients with DM varies from 50% to 70% [1, 2]. Topical antibiotics remain the first-line treatment for bacterial keratitis. Clinicians weigh many factors when choosing an antibiotic regimen, including broad-spectrum coverage, toxicity, availability and cost, and region-specific epidemiology of pathogens and resistance patterns [3]. We believe that, in diabetic mellitus patients with delayed healing of bacterial corneal ulcer, it would be reasonable to use autologous serum eye drops after clinical or laboratory confirmation that the offending microorganisms have been eradicated. Schulze and colleagues [4] demonstrated that, in diabetic patients undergoing pars plana vitrectomy who received corneal abrasion for better intraoperative visualization, autologous serum led to a two times faster re-epithelialization of corneal epithelial wounds after abrasion compared with hyaluronic acid-based artificial tears. The impact of low molecular weight fraction of autologous serum on epithelial regeneration in the therapeutic and surgical management of persistent epithelial defects and ulceration in degenerative and dystrophic corneal disorders has been demonstrated by findings of research that was carried out at the Filatov Institute of Eye Disease and Tissue Therapy [5]. However, to the best of our knowledge, there have been no reports on whether adjunctive autologous serum is efficacious in the comprehensive treatment for bacterial ulcers in patients with DM. The purpose of the study was to determine the impact of adjunctive topical autologous serum eye drops on delayed healing of bacterial corneal ulcers in patients with type 1 diabetes mellitus (DM1). Material and Methods We retrospectively reviewed the results of observing 19 DM1 patients (19 eyes) with bacterial corneal ulcers who exhibited delayed healing of corneal ulceration and whose corneal smears and scrapings taken one week after initiation of treatment were bacteriologically negative. Patient age varied from 18 to 48 years (mean, 32.1±8.9 years). Diabetic polyneuropathy (DPN) was found in all patients. Of the 19 patients, 10 (52.6%) had symptomatic DPN, and 9 (47.4%) had disabling DPN as per the classification of Dyck (1999) [6, 7]. In addition, of the 19 patients, 6 (31.6%) had inadequately controlled diabetes (defined as hemoglobin A1c (HbA1c) level of 7.1-7.5%), and 13 (68.4 %) had out-of-control diabetes (defined as HbA1c ≥7.5%). Moreover, of the 19 patients, 8 (42.1%) had a diabetes duration of 5 to 10 years, and 11 (57.9%) had a diabetes duration of more than 10 years. The study protocol complied with the Declaration of Helsinki and was approved by the Ethics and Bioethics Committee of Kharkiv National Medical University (Meeting Minutes, December 5, 2018). Bacterial keratitis was diagnosed on the basis of typical clinical picture and the diagnosis was bacteriologically confirmed. A single pathogen was found in 17 eyes (89.5%). A Gram positive species was identified in 15 (88.2%) of the 17 eyes, with Staphylococcus epidermidis being the most common (9 eyes; 52.9%), followed by Staphylococcus aureus (3 eyes; 17.6%), Streptococcucus viridans (2 eyes; 11.8%), and Staphylococcus saprophyticus (1 eye; 5.9%). In addition, a Gram negative species was identified in 2 (11.8%) of the 17 eyes (Moraxella species, 1 (5.9%) and Esherichia coli, 1 (5.9%)). Moreover, mixed microflora (Staphylococcus aureus and Staphylococcus epidermidis) was evident in 2 (10.5%) of the 17 eyes. Location of the ulcer defect was central in 5 eyes (26.3%), paracentral in 12 eyes (63.2%), and peripheral in 2 eyes (10.5%). Before being included in the study, DM1 patients with bacterial keratitis were treated with topical antibiotics (specifically, they received topical ofloxacin before the bacteriological results were available, and the antibiotic relevant to the treatment of a particular bacterial infection after the results were available), antiseptics, antioxidants, repairing agents, artificial tears (0.15-0.4% hyaluronic acid), and mydriatics. In addition, systemic anti-inflammatory agents were administered. The bacteriological results (confirming the absence of organisms on microbiologic re-examination of corneal scrapings and smears) were available a week after sending these scrapings and smears to the laboratory, i.e., two weeks after initiation of treatment. At this time point, patients of the control group (9 individuals) continued receiving only background therapy (topical antiseptics, antioxidants, repairing agents, artificial tears, and mydriatics and systemic anti-inflammatory agents), whereas patients of the main group began receiving autologous serum eye drops in addition to background therapy of topical antiseptics, mydriatics and systemic anti-inflammatory agents. Autologous serum was prepared according to a procedure reported by Geerling and colleagues [6]. Venesection was performed at the antecubital fossa under aseptic conditions and 50-100 ml of whole blood was collected into sterile containers. The containers were left standing for 2 hours at room temperature (18-25 °C) in an upright position to ensure complete clotting before they were spun in a laboratory centrifuge at 3000 g for 15 minutes. The supernatant serum was filtered without dilution through a 0.2 μm filter under sterile conditions, and portions of 1-2 ml were aliquoted into sterile test tubes. The tubes were sealed and labeled. Following this protocol, 50 ml of whole blood yielded 20–25 ml of serum. Patients were instructed to store the tubes frozen at -20 °C until needed, and to thaw a tube at + 4 °C. A thawed tube had to be discarded at the end of the day. Autologous serum eye drops were instilled every 2 hours during wakefulness until the corneal defect healed. Thereafter, they were instilled 4 times daily during a week or until serum exhausted [7]. Patients of the main group and controls were comparable with regard to age, diabetes control and duration, and severity of DPN and clinical symptoms. Patients had not only a routine eye examination (visual acuity, tonometry, and slit-lamp biomicroscopy of anterior and posterior eye segments), but also specific eye studies, including bacteriological studies, fluorescein dye test, anterior eye OCT (TOPCON 3D OCT-2000) and non-contact corneal esthesiometry. Non-contact corneal esthesiometry was performed with the use of the device we have developed for this purpose [8]. Corneal sensation was assessed at nine specified examination points (superior, superior nasal, superior temporal, nasal, central, temporal, inferior nasal, inferior temporal, and inferior points), and average corneal sensitivity threshold was determined. The following parameters for the novel non-contact air-jet corneal esthesiometer were used: diameter of air jet output orifice, 0.5 mm; pulse duration, 1 s; distance to the corneal surface, 4 mm; and air jet temperature, 20 °С. The minimum air jet force was used initially. Thereafter, the air jet force was gradually increased until the subject reported a sensation of breeze. The parameters for assessment of changes in bacterial corneal ulcers were scored as follows: pericorneal injection (1: mild; 2: moderate; 3: marked); size of ulcer defect (1: < 2 mm; 2: 2-5 mm; 3: >5 mm); OCT-measured depth of the corneal defect (1: < 1/3 of the corneal thickness; 2: 1/3-2/3 of the corneal thickness; 3: >2/3 of the corneal thickness); edema of the corneal tissue surrounding the ulcer (1: epithelial; 2: stromal; 3: diffuse); corneal infiltration (1: epithelial; 2: stromal; 3: diffuse); and reduction in corneal sensitivity (1: mild, 80-130 mL/min; 2: moderate, 130-150 mL/min; 3: marked, >150 mL/min). Clinical picture data for day 1 (visit 1, before administering the treatment) and days 7 (when corneal samples and scrapings were re-taken), 14 (when bacteriology results became available and main group patients were additionally administered autologous serum), 17, 21 and 24 was analyzed. Mean and standard deviation (SD) values as well as ranges for corneal sensitivity threshold were calculated. The Mann-Whitney rank test was used to compare the scores for pericorneal injection, size and depth of the corneal defect, edema of the corneal tissue surrounding the ulcer, corneal infiltration, and reduction in corneal sensitivity for patients of the serum group and controls. The level of significance p ≤ 0.05 was assumed. Excel 2010 was used to develop the primary data base, and Stata 12 software (Stata Corp., College Station, TX) was used for statistical analyses. Results In the main group, a reduction in pericorneal injection compared with the control group was noted as early as day 3 of receiving autologous serum eye drops (day 17 of the study) (p < 0.05), and conjunctival color appeared to normalize in all patients of the group on day 10 (Table 1). However, mild pericorneal injection was still present in 33.3% of the controls on day 24.
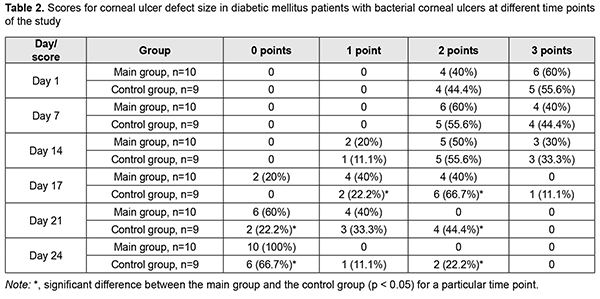
A reduction in corneal defect size compared with the control group was noted on day 3 (p < 0.05), and the wound fully epithelialized on day 10 of receiving autologous serum eye drops in all patients of the main group (Table 2). An ulcer defect was still present in 33.3% of the controls on day 24; the defect sized less 2 mm in 11.1%, and 2-5 mm in 22.2% of the controls.
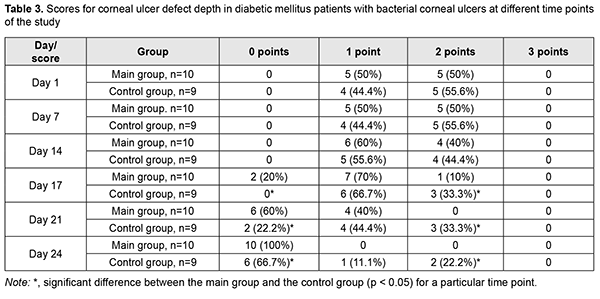
Ulcer defect was shallow compared to controls in most main group patients on day 3 (p < 0.05), and fully epithelialized on day 10 of receiving autologous serum eye drops in all patients of the main group (Table 3). An ulcer defect was still present, and was as deep as less than 1/3 of the corneal thickness in 11.1%, and as deep as 1/3-2/3 of the corneal thickness, in 22.2% of the controls on day 24.
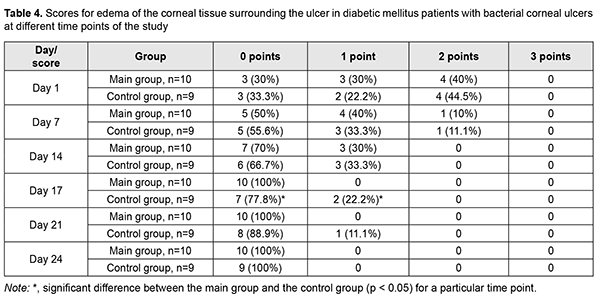
Edema of the corneal tissue surrounding the ulcer completely resolved in all patients of the main group on day 17 after visit 1 (i.e., day 3 of receiving autologous serum eye drops), and in controls, on day 24 (Table 4). In addition, edema of the corneal tissue surrounding the ulcer was still present in 22.2% of the controls on day 17, and in 11.1% of the controls on day 21.
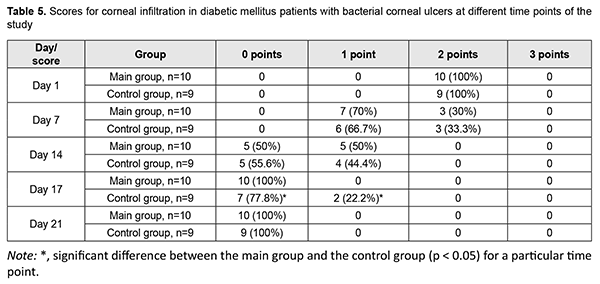
Corneal infiltration resolved in all patients of the main group on day 17 after visit 1, and in controls, on day 21 (Table 5). On day 17, corneal epithelial infiltration was still present in 22.2% of the controls.
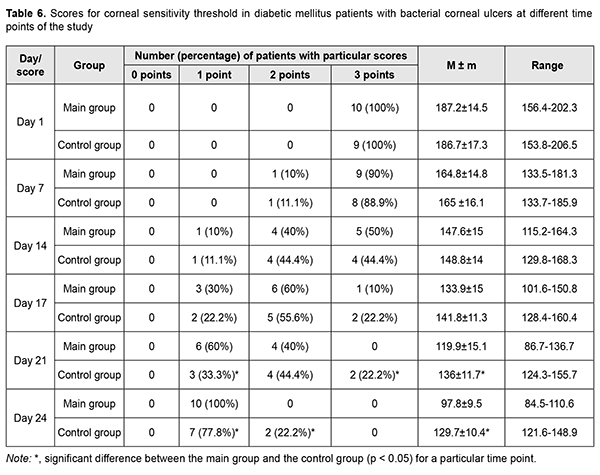
Compared to controls, patients of the main group showed 13.4% and 32.6% lower mean corneal sensitivity threshold on day 7 and day 10 of receiving autologous serum eye drops (i.e., day 24 of the study), respectively (Table 6). At visit 1, severely decreased corneal sensitivity was noted in all patients of both groups. On day 14 of the study, severely, moderately and mildly decreased corneal sensitivity was found in 5 (50%), 4 (40%) and 1 (10%) patients, respectively, of the main group, versus 4 (44.45%), 4 (44.45%) and 1 (11.1%) patients, respectively, of the controls, with no significant difference between groups. On day 24 of the study, all patients of the main group showed mildly decreased corneal sensitivity, and 2 (22.2%) and 7 (77.8%) of the control group, moderately and mildly decreased corneal sensitivity, respectively.
Therefore, on day 10 of receiving autologous serum eye drops (day 24 of the study), corneal ulcer was found to be healed in all patients of the main group versus 66.7% of the control group. In addition, of patients of the control group, 11.1% showed central corneal defects sized less than 2 mm and as deep as less than 1/3 of the corneal thickness, and 22.2% showed paracentral corneal defects sized 2-5 mm and as 1/3-2/3 of the corneal thickness, and these defects were accompanied by mild pericorneal injection. This was the reason why three patients of the control group were recommended to have surgery, specifically, therapeutic keratoplasty. Bacterial corneal ulcer resulted in nubecula corneae and macula corneae in 5 (50%) and 5 (50%), respectively, patients of the main group (controls: 4 (44.4%) and 2 (22.2%), respectively). Therefore, in DM1 patients with delayed healing of bacterial corneal ulcer, the use of adjunctive autologous serum eye drops after eradication of the offending microorganisms resulted in reductions in precorneal injection and size and depth of the ulcer defect and complete resolution of corneal infiltration and edema of the corneal tissue surrounding the ulcer as early as day 3, and recovery of all patients and a 32.6% improvement in corneal sensitivity on day 10. Discussion Corneal inflammation is more common and runs a more severe course in patients with diabetic mellitus than in general population [1]. Diabetes mellitus is a risk factor for developing bacterial keratitis, but has no effect on the incidence of fungal and amoebic keratitis [9]. This might be associated with increased presence of Gram-positive species (mostly, coagulase-negative staphylococci) in the conjunctival flora due to increased tear glucose levels in and frequent use of antibiotics by patients with diabetes mellitus [10]. Severity of bacterial keratitis associated with diabetes mellitus may result from the presence of diabetic keratopathy, i.e., structural and functional corneal changes developing in diabetic patients in the presence of diabetic corneal neuropathy [1]. Numerous authors propose to use autologous serum in the form of eye drops as adjuvant therapy for various ocular conditions, including dry eye, persistent epithelial defects, and superficial limbic keratoconjunctivitis, and to promote engraftment after penetrating keratoplasty [4-7,11]. Of note is that autologous serum normalizes healing of corneal epithelial abrasions in diabetic patients undergoing vitrectomy [4]. The mechanism of action of autologous serum is believed to be associated with its components, growth factors (like epidermal growth factor, transformation growth factor (TGF) beta, and platelet-derived growth factor), vitamins A and E, neurotrophic factors, fibronectin, immunoglobulins, cytokines and bacteriostatic]. In conclusion, we found adjunctive topical autologous serum eye drops to be efficacious in the comprehensive treatment of delayed healing of bacterial corneal ulcer in patients with DM1 after eradication of the offending microorganisms, resulting in reductions in precorneal injection and size and depth of the ulcer defect and complete resolution of corneal infiltration and edema of the corneal tissue surrounding the ulcer as early as day 3, and recovery of all patients and a 32.6% improvement in corneal sensitivity on day 10.
References 1.Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. Biomed Res Int. 2016; 2016:3801570. 2.Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005; 89:254–5. 3.Austin A, Lietman Т, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017:124(11):1678-89. 4.Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142(2):207–11. 5.Troichenko LF. [Efficacy of the improved method for treating persistent epithelial defects and torpid ulcers of the cornea]. Cand Sc (Med) Thesis. Odesa: Filatov Institute of Eye Disease and Tissue Therapy. 2013. Ukrainian. 6.Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004 Nov;88(11):1467-74. 7.Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. Biomed Res Int. 2013;2013:521315. 8.Zavoloka OV, Besditko PA, Lukhanin OO. Efficacy of a novel non-contact corneal esthesiometer in assessing the neurotrophic status of the cornea in type I diabetic patients with bacterial keratitis. J Ophthalmol (Ukraine). 2019;6:29-33. 9.Wang B, Yang S, Zhai H, Zhang Y, Cui C, Wang J, Xie L. A comparative study of risk factors for corneal infection in diabetic and non-diabetic patients. Int J Ophthalmol. 2018;11(1):43–7. 10.Grzybowski A, Kanclerz P, Huerva V, Ascaso F, Tuuminen R. Diabetes and Phacoemulsification Cataract Surgery: Difficulties, Risks and Potential Complications. J Clin Med. 2019;8(5):716. 11.Pflugfelder SC. Is autologous serum a tonic for the ailing corneal epithelium? Am J Ophthalmol. 2006 Aug;142(2):316-7.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|