J.ophthalmol.(Ukraine).2020;5:21-28.
|
http://doi.org/10.31288/oftalmolzh202052128 Received: 05 August 2020; Published on-line: 27 October 2020 Pupil response to accommodation and convergence in children and adolescents with accommodative disorders Shakir Dukhayer, N. M. Bushuieva, S. B. Slobodianyk SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: bushuyevan@gmail.com TO CITE THIS ARTICLE:Dukhayer Shakir, Bushuieva NM, Slobodianyk SB. Pupil response to accommodation and convergence in children and adolescents with accommodative disorders. J.ophthalmol.(Ukraine).2020;5:21-8. http://doi.org/10.31288/oftalmolzh202052128 Background: As there is close association between pupil response and accommodation during gaze fixation on an object at any distance, it seems interesting to study pupil response to accommodation in patients with accommodative disorders. Purpose: To assess pupil response to accommodation and convergence in children and adolescents with accommodative disorders who varied in age and autonomic nervous system (ANS) tone. Material and Methods: One hundred and thirty children and adolescents (260 eyes) having accommodation spasm (AS) and 59 (118 eyes) having accommodative insufficiency (AI) were included in the study. They underwent measurements of best-corrected visual acuity, refractive errors, and axial length, and assessment of accommodation reserve (by the technique of Dashevsky) and body’s autonomic nervous system (ANS) balance (by Kerdo index). Pupillography studies were performed using an OK-2 pupillographer (Ukraine) to assess pupil area, amplitude of change in pupil area, and durations of phases of changes in pupil area. Results: An increased parasympathetic tone was more common among 6-14-year-old children with AS than among age-matched healthy children (64.5% vs 29-43%), and the rest of these cases had an increased sympathetic tone. The mean maximum pupil area Smax value for children with AS and increased sympathetic tone was 27.5 ± 4.1 mm2, which was 25.6% greater than for those with AS and increased parasympathetic tone (21.9 ± 5.6 mm2, p < 0.001), and 102% less than for healthy children with increased sympathetic tone (55.6 ± 13.5 mm2, p < 0.001). In addition, pupil constriction latency (Phase II) and active pupil constriction (Phase III) were longer for patients than for controls. Among children and adolescents with AS, pupil parameters significantly depended on the ANS tone, but not on the age. This was different from healthy children, in whom pupil area parameters correlated both with age and with ANS tone, whereas durations of changes in pupil area (i.e., phases III, V and VI) were correlated only with age. All children with AI had an increased sympathetic tone. Compared to healthy children with an increased sympathetic tone, children with AI demonstrated smaller Smax and longer pupil re-dilation latency (Phase V) (p < 0.05). There was no significant difference in other pupil characteristics between patients of the study and healthy individuals. We found a substantial difference in Smax between children with AS (23.94±5.5 mm2) and those with AI (49.3 ± 13.8 mm2). Conclusion: Children with AS develop some imbalance of the body’s and ocular autonomic nervous systems with a shift towards an increased parasympathetic activity. The relationships developed between pupil responses, age and state of autonomic innervation in children with AS are different from those in healthy children, with the tone of the ANS, but not age playing a major role. We found a substantial difference in Smax between children with AS and those with AI, which may be considered along with clinical signs and body’s ANS tone as differential diagnostic criteria for accommodative disorder and facilitate a pathogenesis-oriented treatment approach. Keywords: accommodation spasm, accommodative insufficiency, pupillography, pupil response to accommodation and convergence, children and adolescents, autonomic nervous system
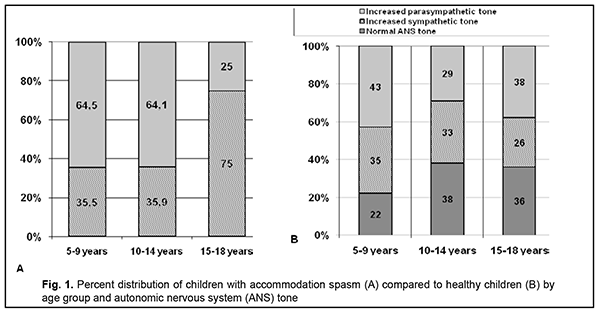
Introduction Accommodation is closely associated with pupil response; both of them are components of the uncontrolled reflex which is implemented through the activity of the accommodative and convergence system. The system provides simultaneous achievement of best-corrected visual acuity, binocular vision, and correct position of the eyes focused on objects at any distance [1]. The link between accommodation and pupil response becomes apparent when the gaze is shifted from a distant object to a near object, and the pupil constricts concurrent with an increase in accommodation and convergence, i.e., pupil response to accommodation and convergence develops. Such synchronicity is achieved through autonomic co-innervation of the ciliary and iris muscles, which results from simultaneous interaction of the autonomic sympathetic and parasympathetic nervous systems [1-3]. As there is a close association between pupil response and accommodation during gaze fixation on an object at any distance, it seems interesting to study pupil response to accommodation as a potential objective criterion for assessing the function of the accommodative and convergence system both in healthy individuals of any age and in those with accommodative disorders. We believe that this task is important since the incidence of accommodative impairment significantly increases with a rapid increase in computerization, visual load, distant education and work. The purpose of the study was to assess pupil response to accommodation and convergence in children and adolescents with impaired accommodation who varied in age and autonomic nervous system tone. Material and Methods Pupil response to accommodation and convergence was studied in 189 children and adolescents with accommodative impairments aged 6 to 18 years. Of these, 130 children and adolescents (260 eyes) had accommodation spasm and 59 (118 eyes) had accommodative insufficiency. The data from our previous pupillography study [4] of 269 systemically and ophthalmologically healthy children and adolescents (538 eyes) aged 7 to 18 years were used as normative data for comparison. Subjects were divided into three groups based on their age: group 1 (6 to 9 years), group 2 (10 to 14 years), and group 3 (15 to 18 years). They underwent an eye examination that included measurement of best-corrected distance visual acuity (Sivtsev chart) and near visual acuity, autorefractometry, assessment of accommodation reserve (by the technique of Dashevsky) and axial length measurement by ultrasound. All children with accommodation spasm (or аccommodative excess [5, 2] complained of reduced distance vision (but not of reduced near vision); daily fluctuations in visual acuity; and visual fatigue. In addition, some of them complained of a headache. In all patients with accommodation spasm, uncorrected visual acuity (UCVA) was lower than normal for their age group, best-corrected visual acuity (BCVA) was 1.0 or better, and accommodation reserve (as assessed by the technique of Dashevsky) was 1.5 D or less. Most cases (95.7%) had myopic refraction (-3.0 D or less; mean value, 1.0 ± 0.63 D), and the rest (4.4%) had emmetropic refraction. Mean axial length was 23.7 ± 0.73 mm. Children with clinical signs of accommodative insufficiency complained of reduced vision (a reduction in vision was more pronounced at near than at distance) and visual fatigue. In all patients with accommodative insufficiency, UCVA was lower than normal for their age group, BCVA was 1.0, and accommodation reserve (as assessed by the technique of Dashevsky) was 1.5 D or less. The autonomic nervous system balance was assessed by Kerdo index (KI) [6]. KI = 1 – D/P, where D is diastolic blood pressure (expressed in mmHg) and P is cardiac frequency (or heart rate, expressed in beats/min). KI equals zero in normal autonomic nervous system tone, is negative in individuals with an increased parasympathetic tone (parasympathotonia), and positive in those with an increased sympathetic tone (sympathotonia) of the autonomic nervous system. Pupillography studies were performed using an OK-2 pupillographer (Ukraine) [7, 8] developed by the Filatov institute in cooperation with Om-Tekhnologiia (Odesa, Ukraine). Pupillometry studies of pupil response to accommodation and convergence were conducted using our methodology reported previously [4, 7, 8]. The subject fixed his eyes on a test object located at a distance of 100 cm (relaxed accommodation and convergence) and shifted his gaze to a test object located at a distance of 10 cm (strained accommodation and convergence). Changes in pupil parameters during pupil response to accommodation and convergence were recorded. The following pupillography indices were subjected to analysis: maximum pupil area (Smax, under relaxed accommodation), minimum pupil area (Smin, under strained accommodation), amplitude of change in pupil area (A), and duration of phases of change in pupil size (Phase II, pupil constriction latency; Phase III, active pupil constriction; Phase V, pupil re-dilation latency; and Phase VI, fast pupil re-dilation). Statistical analyses were conducted using Statistica 8 (StatSoft, Tulsa, OK, USA) and Microsoft Excel 2007 software. Mean values, standard deviations (SD), and their 95% confidence intervals and significance values (p) were calculated. The Student t-test was used to determine the significance of normally distributed parametric values. The Wilcoxon-Mann-Whitney was used for data that were not normally distributed. The level of significance p < 0.05 was assumed. The non-parametric Spearmen test was used to calculate correlation coefficients; these were considered significant at p < 0.05 [9, 10]. Results At baseline, ANS balance was assessed using the Kerdo index. Figure 1 shows distribution of children with accommodation spasm vs healthy children in terms of age and ANS tone. In 6-14-year-old children with accommodation spasm, an increased parasympathetic tone was the most common (64.5%), followed by an increased sympathetic tone (35.5%), but there were no cases with normal ANS tone.
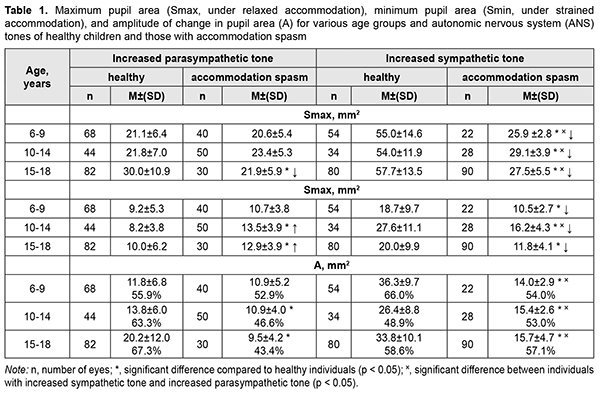
Increased parasympathetic tone was more common among 6-14-year-old children with accommodation spasm than among age-matched healthy children (64.5% vs 29-43%). Of the 15-18-year-old adolescents, 75% exhibited increased sympathetic tone, and 25%, increased parasympathetic tone. In children with accommodation spasm and increased parasympathetic tone, maximum pupil area (Smax, under relaxed accommodation) did not depend on age and was practically similar for all the three age groups (mean Smax, 21.9 ± 5.6 mm2; Table 4.2). In 6-14-year-old children with accommodation spasm and increased parasympathetic tone, the mean Smax value was practically the same as that for their age-matched controls. However, in 15-18-year-old adolescents with accommodation spasm and increased parasympathetic tone, the mean Smax value was 27% less than for their age-matched controls (21.9 ± 5.9 mm2 vs 30.0 ± 10.9 mm2, p < 0.05). The mean Smax value for children with accommodation spasm and increased sympathetic tone was 27.5 ± 4.1 mm2, which was 25.6% greater than for those with accommodation spasm and increased parasympathetic tone (21.9 ± 5.6 mm2, p < 0.001), and 102% less than for healthy children with increased sympathetic tone (55.6 ± 13.5 mm2, p < 0.001). These findings may indicate that in children with accommodation spasm and increased sympathetic tone, the sympathetic effect on the pupil is less pronounced than in healthy children with increased sympathetic tone, likely due to significantly locally increased parasympathetic component of the balance of autonomic innervation. The minimum pupil area Smin (under strained accommodation) was practically similar for children with accommodation spasm and various ANS tones, with a mean value of 11.8±3.8 mm2 (Table 1). However, 10-14-year-old children with increased sympathetic tone exhibited somewhat greater Smin values (16.2 ± 4.3 mm2; p < 0.05) than others.
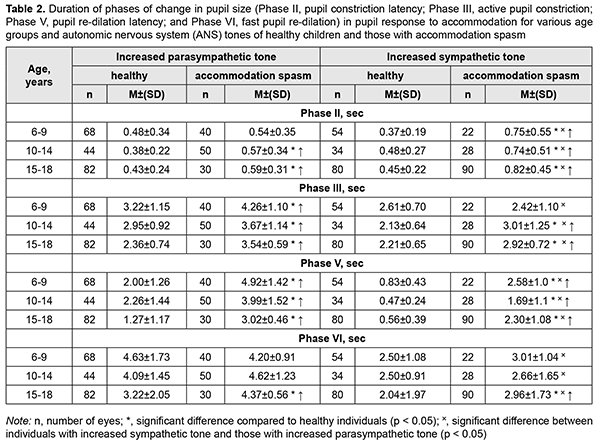
The mean Smin value for children with accommodation spasm and increased sympathetic tone was 41-44% less than for healthy children with increased sympathetic tone (p < 0.01). However, the mean Smin value for 6-9-, 10-14-, and 15-18-year-old patients with accommodation spasm and increased parasympathetic tone was 16%, 65%, and 29%, respectively, greater, than for age-matched controls (p < 0.05). Compared to healthy children, most children with accommodation spasm exhibited significantly lower amplitude of change in pupil area (A) during accommodative response (p < 0.001). This was particularly relevant to children with increased sympathetic tone, who exhibited a reduction of 42-61%. In addition, there was a significant difference in the absolute value of A between patients with increased parasympathetic tone and those with sympathetic tone (10.6±4.5 mm2 vs 15.5±3.4 mm2, respectively, p < 0.05), but not between patients of various age groups. However, the percentage ratio of amplitude of change in pupil area (A) to baseline pupil area (Smax) was practically similar for children with various ANS tones, and varied from 43.4% to 54.3%. Of general note is that, compared with healthy individuals, children with accommodation spasm exhibited a tendency to lower percentage ratio of A to Smax. Table 2 shows durations of phases of change in pupil size (Phase II, pupil constriction latency; Phase III, active pupil constriction; Phase V, pupil re-dilation latency; and Phase VI, fast pupil re-dilation) during the accommodative response for healthy children and those with accommodation spasm.
There was a significant difference in the pupil constriction latency (Phase II) between patients with increased parasympathetic tone and those with sympathetic tone (0.57±0.27 s vs 0.77 ± 0.50 s, respectively, p < 0.05), but not between patients of various age groups. The mean Phase II for 10-14- and 15-18-year-old patients with accommodation spasm and increased parasympathetic tone was 50%, and 37%, respectively, longer (p < 0.05), and for 6-9-, 10-14-, and 15-18-year-old patients with accommodation spasm and increased sympathetic tone, 102%, 54%, and 82%, respectively, longer (p < 0.05), than for age-matched normal controls. In addition, the mean Phase III (active pupil constriction) for patients with accommodation spasm and increased parasympathetic tone was 24%-50% longer (p < 0.01), and for patients with accommodation spasm and increased sympathetic tone, 32%-41%, longer (p < 0.01), than for age-matched normal controls. The mean Phase III duration for patients with accommodation spasm and increased parasympathetic tone was 3.82 ± 0.94 s, and for those with increased sympathetic tone, 2.78 ± 1.02 s (p < 0.01) (Table 2). Moreover, the mean Phase V (pupil re-dilation latency) for 6-9-, 10-14-, and 15-18-year-old patients with accommodation spasm and increased parasympathetic tone was 150%, 77%, and 138%, respectively, longer (p < 0.01), and for those with increased sympathetic tone, 210%, 260%, and 311%, respectively, longer (p < 0.01), than for age-matched normal controls. Among patients with accommodation spasm, the Phase VI (fast pupil re-dilation) did not depend on age and was practically the same as for normal controls (Table 4.6). The mean Phase VI duration for patients with accommodation spasm and increased sympathetic tone was 2.88 ± 1.47 s, which was 65% shorter, than for those with increased parasympathetic tone 4.40 ± 0.9 с (p <0.01). Among children and adolescents with accommodation spasm, pupil parameters significantly depended on the ANS tone, but not on the age (Table 3). Among healthy children, pupil area parameters (Smaх, Smіn, and A) correlated with the ANS tone, whereas durations of phases III, V and VI were negatively correlated with age, but did not correlate with the ANS tone (Table 3).
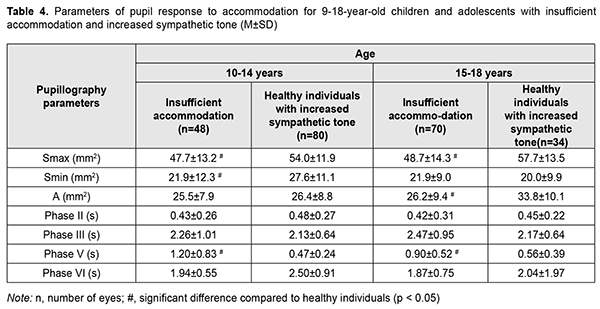
There were two age groups of children with accommodative insufficiency (9-14 years and 14-18 years, respectively). All children with accommodative insufficiency had an increased sympathetic tone. Table 4 shows values of the parameters of accommodative response of the pupil for these children and adolescents. Pupillography parameters for accommodative insufficient children were practically similar for both age groups, and there was a difference only in some parameters between accommodative insufficient children and healthy children. Thus, compared to healthy children with increased sympathetic tone, children with accommodative insufficiency demonstrated smaller maximum pupil area and longer pupil re-dilation latency (Phase V) (p < 0.05).
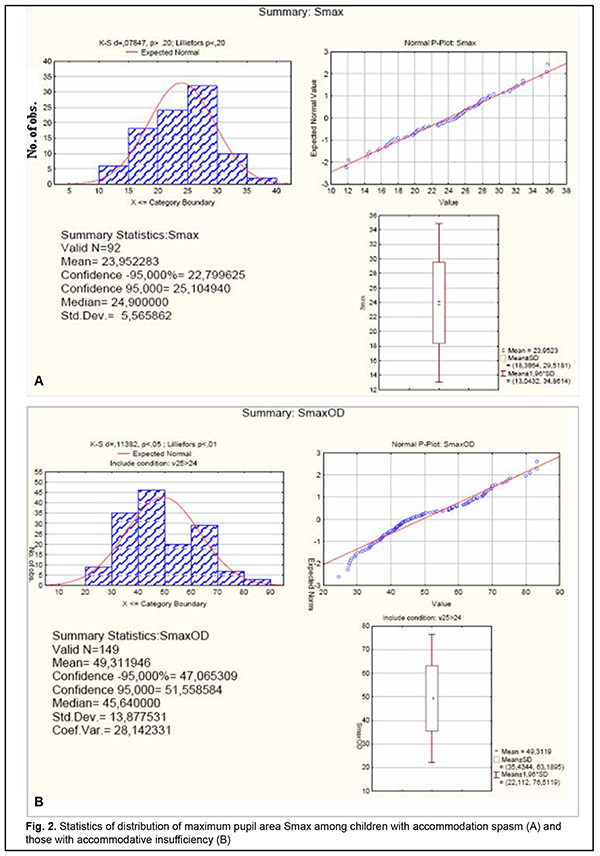
There was a significant difference in maximum pupil area (Smax) between children with accommodation spasm and accommodative insufficient children (Fig. 2).
The Smax for children with accommodation spasm (both for those with increased sympathetic tone and for those with increased parasympathetic tone) was significantly smaller than the norm, with a mean value of 23.94±5.5 mm2 (95% CI, 22.8 – 25.0). However, the Smax for accommodative insufficient children with increased sympathetic tone (mean value, 49.3±13.8 mm2; 95% CI, 47.1–51.6) was twice as great as for children with accommodation spasm and was close to the value for healthy children with increased sympathetic tone. Discussion Our finding of a high incidence of increased parasympathetic ANS tone among 6-14-year-old children with accommodation spasm compared to normal controls (64% vs 29-43%) indicates increased parasympathetic effects in children of this age group developing spasm of accommodation. Although an increased sympathetic tone was predominant at the level of the body in 75%, and an increased parasympathetic tone was predominant at the level of the body in 25% of adolescents of 15-18 years, an increased parasympathetic tone (as assessed by the pupil area) was predominant at the level of the eye in all patients of this age group. Therefore, one may suppose that children with accommodation spasm develop some imbalance of the body’s and ocular autonomic nervous systems with a shift towards a predominant parasympathetic activity; there are, however, contradictory reports on this matter in the literature. Thus, in a study by Volkova [11], of the total 6-22- year-old mild or moderate myopes with accommodative disorders, 28% exhibited increased parasympathetic tone; 23.4%, increased sympathetic tone; and 48.6%, normal ANS tone. In addition, the pupil diameter in myopes depended on the autonomic nervous system tone, and myopes with an increased sympathetic tone exhibited larger pupil diameter (5.33-5.88 mm) than those with normal ANS tone (4.28-4.33 mm) or those with an increased parasympathetic tone (3.88-4.08 mm). Moreover, myopes with an increased parasympathetic tone exhibited more substantial reductions in relative accommodation reserve and range of distant accommodation. On the basis of these findings, Volkova [11] hypothesized that an increased parasympathetic tone contributes to the development of accommodative spasm, whereas reduced distance accommodation volume indicates a paresis of distance accommodation. In a study by Prediger [12], of the total 10-16-year-old children with acquired myopia, 75% exhibited an increased sympathetic tone (as assessed by Kerdo index). However, an increased parasympathetic tone was predominant in the eye, which made the author define the local ANS tone as parasympathetic. In the current study, we found that patients with accommodation spasm had an increased local parasympathetic effect on the pupil, irrespective of the body’s ANS tone, which was confirmed by reduced maximum pupil area (Smax) and longer pupil re-dilation latency (Phase V) compared to the norm. Children with accommodation spasm and an increased sympathetic tone showed the most pronounced local parasympathetic effects, with a 128% reduction in maximum pupil area (Smax) compared to healthy children with an increased sympathetic tone. Of note is also a significantly longer duration of Phase II (pupil constriction latency) and Phase III, (active pupil constriction) in patients with accommodation spasm compared to healthy children, which may indicate a delay in nerve impulse transmission to effectors of the pupil response to accommodation and convergence due to local ANS dysfunction. Therefore, our findings confirm the presence of locally increased parasympathetic tone, which is a characteristic attribute of all patients with accommodation spasm irrespective of their body’s ANS tone. In addition, we found pupillary parameters to correlate with ANS tone, but not with age in children with accommodation spasm. This was different from healthy children, in whom pupil area parameters (Smaх, Smіn, and A) correlated both with age and with ANS tone, whereas durations of changes in pupil area (i.e., phases III, V and VI) were correlated only with age. Therefore, the relationships developed between pupil responses, age and state of autonomic innervation in children with accommodation spasm are different from those in healthy children, with the tone of the ANS, but not age playing a major role. All children with accommodative insufficiency exhibited an increased sympathetic tone as assessed by Kerdo index. In addition, their pupillography parameters did not depend on age, and maximum pupil area (Smax) was smaller, whereas pupil re-dilation latency (Phase V) was longer than those for healthy children with an increased sympathetic tone (p < 0.05). Moreover, we found a substantial difference in Smax between children with accommodation spasm (23.94±5.5 mm2 [95% CI, 22.8 – 25.0]) and those with accommodative insufficiency (49.3 ± 13.8 mm2 [95% CI, 47.1– 51.6]), which may be considered along with clinical signs and body’s ANS tone as differential diagnostic criteria for accommodative disorder and facilitate a pathogenesis-oriented treatment approach. References 1.Von Noorden GK, Campos EC. Binocular Vision and Ocular Motility. Theory and management of strabismus. 6th ed. St. Louis, MO: Mosby; 2001. 2.Katargina LA, editor. [Accommodation: A guide for physicians]. Moscow: Aprel; 2012. Russian. 3.Miller NR, Newman NJ, Biousse V, Kerrison JB. Walsh & Hoyt’s Clinical Neuro-ophthalmology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. 4.Dukhayer Shakir, Bushuieva NM, Slobodianyk SB. Pupil response to accommodation and convergence in healthy children and adolescents of various age groups and autonomic nervous system tone. J Ophthalmol (Ukraine). 2020;2:37-45. 5.Emslie R., Claassens А., Sachs N., Walters I. The near triad and associated visual problems. S Afr Optom. 2007; 66(4): 184-191. 6.Vein AM, editor. [Autonomic nervous system abnormalities: Clinical signs, diagnosis and treatment]. Moscow: Meditsinskoe informatsionnoe agenstvo; 2003. Russian. 7.Bushuieva NM, Boychuk IM, Dukhayer Shakir, Khramenko NI, Ponomarchuk VS. [Method of computer pupillography]. Ukrainskyi medychnyi almanakh. 2006;9(2):24-7. Ukrainian. 8.Bushuieva NM, Boychuk IM, Khramenko NI. [Method for diagnosing disorders of accommodation based on pupillographic studies of pupillary response]. Arkhiv klinicheskoi i eksperimentalnoi meditsiny. 2001;10(2);132-3. Russian. 9.Glanz S. [Biomedical statistics]. Moscow: Praktika;1998. Russian. 10.Zaitsev VM, Lifliandskii VG, Maeinkin VI. [Tutorial in applied medical statistics]. St Peterburg:Foliant;2008. Russian. 11.Volkova EM. [Effect of accommodative nervous system tone on accommodation function in myopia]. Abstract of Cand Sc (Med) Thesis. Yaroslavl: Yaroslavl State Medical Academy; 2007. Russian. 12.Prediger VM. [Clinical, functional and neurophysiological assessment of children with acquired myopia]. Abstract of Cand Sc (Med) Thesis. Novosibirsk: Novosibirsk State Medical University; 2017. Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|