J.ophthalmol.(Ukraine).2020;5:8-12.
|
http://doi.org/10.31288/oftalmolzh20205812 Received: 09 April 2020; Published on-line: 26 October 2020 Pseudoexfoliative glaucoma: clinical signs Iryna M. Bezkorovayna, Jouini Dhia Eddine, Nina M. Bezega Ukrainian Medical Stomatological Academy; Poltava (Ukraine) E-mail: ibezkor@gmail.com TO CITE THIS ARTICLE: Bezkorovayna IM, Jouini Dhia Eddine, Bezega NM. Pseudoexfoliative glaucoma: clinical sign. J.ophthalmol.(Ukraine).2020;5:8-12. http://doi.org/10.31288/oftalmolzh20205812 Background: Knowing characteristic clinical signs of pseudoexfoliative glaucoma (PEG) is important for a practicing ophthalmologist because it would allow early detection of a particularly severe form of glaucomatous process in the presence of pseudoexfoliative syndrome (PXS). PEG accounts for 25%-30% of all open-angle glaucoma cases. Most patients with PXS undergo cataract surgery with undiagnosed PEG, and, in these cases, glaucoma is late detected and misclassified as conventional open-angle glaucoma. Purpose: To determine characteristic clinical PEG signs that could be used as diagnostic aids. Material and Methods: One hundred and fourteen patients (146 eyes; age, 59 to 90 years; mean age, 74.73 ± 0.50 years) with PXS were included in the study. Patients underwent visual acuity assessment, biomicroscopy, gonioscopy, static automated perimetry, and SD OCT (Topcon 3D OCT-2000FA Plus; version 7/21/003/0) using 3D Macula, 3D Disc and 3D (V) Glaucoma Analysis in the Macula 3D (V) over a 7.0x7.0-mm scan area. Results: Signs of the presence of PXS with deposits of exfoliation material in various anterior segment structures were identified in study patients. High intraocular pressure (IOP) levels ranging from 29 mmHg to 34 mmHg were characteristic for the patients with a narrowed or closed anterior chamber angle, increased pigmentation and/or exfoliative conglomerates. Patients with small granular or lace-like exfoliations had an IOP of 22-28 mmHg. Static perimetry showed specific changes in the visual field like a diffuse reduction of MD (48.2% of eyes), expanded blind spot (5.5%), paracentral scotoma (4.0%), nasal step defect (5.5%), focal non-specific defects (13.2%) and concentric visual field narrowing (23.6%). OCT showed thinned NFL (33.60 ± 0.43 in eyes with PXS even without increased IOP, with a mean cup-disc ratio of 0.49 ± 0.04, and a significantly thinned NFL of 28.80±0.42 in eyes with PEG) and NFL+GCL+IPL (98.80±0.43 in eyes with PXS and 84.0±1.87 in PEG). In addition, OCT showed significant inferior, temporal, nasal and total RNFL thinning in eyes with PXS, and even more substantial thinning, in eyes with PEG. This indicated less apparent not only macular but also diffuse peripapillary retinal thinning. Moreover, the retinal thickness values for eyes with PXS were close to those characterizing the presence of glaucomatous process in eyes with open-angle glaucoma. Conclusion: The presence of pseudoexfoliative syndrome is a factor with the threat of PEG (26% of patients with PXS were found to have PEG). The 24-hour IOP curve was characterized by higher IOP values in the morning hours for 28.8% of eyes with PEG, or the late evening and early morning hours, with normal IOP values in the day hours for 71.2% of eyes with PEG. This makes specific monitoring relevant. Retinal NFL and RNFL thickness monitoring in eyes with PXS may contribute to early identification of eyes at risk for PEG. Keywords: pseudoexfoliative syndrome, pseudoexfoliative glaucoma
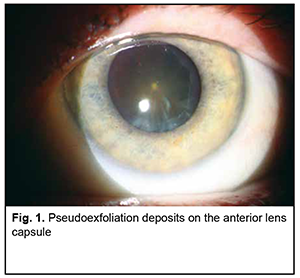
Introduction Pseudoexfoliative glaucoma (PEG) is a particularly severe form of secondary glaucoma which is difficult to diagnose. As PEG accounts for 25%-30% [1] (in our previous study, 26%) of all open-angle glaucoma cases, it is a medical and social challenge [2]. Others and we have reported previously that PEG and cataract frequently run together [1, 2, 3], and the former masquerades as the latter, which makes diagnosing the onset of glaucomatous process difficult. PEG is secondary glaucoma developing due to the susceptibility to pseudoexfoliative syndrome, with accumulation of pigmented and abnormal material from the basement membrane of the anterior segment of the eye in the trabecular meshwork [4]. Pseudoexfoliative damage along with damage to the anterior segment of the eye directly affects nerve fibers, leading to signs of early specific changes [5]. Therefore, if PEG is caused by pseudoexfoliative syndrome (PXS), all patients with PXS should be examined in detail for clinical signs of PEG [6]. However, identifying initial symptoms of PEG is a challenge for practicing ophthalmologists. Clear identification of characteristic clinical signs of PEG should be early and sound. The purpose of this study was to determine characteristic clinical PEG signs that could be used as diagnostic aids. Material and Methods This study was conducted at the Department of Otorhinolaryngology and Ophthalmology, Ukrainian Medical Dentistry Academy, at the premises of the Department of Ophthalmology, Poltava Region Clinical Hospital. The study adhered to the tenets of the Declaration of Helsinki. One hundred and fourteen patients (146 eyes; age, 59 to 90 years; mean age, 74.73 ± 0.50 years; percentages of men and women, 42% and 58%, respectively) with PXS were included in the study. Of these, 29 patients (38 eyes) were diagnosed with PEG. Exclusion criteria were neurologic disorders, age-related macular degeneration, myopia exceeding 3 D, and history of intraocular surgery. Intraocular pressure (IOP) was measured with non-contact pneumotonometry (Topcon CT-80; Japan). In addition, patients with IOP over 21 mmHg underwent hourly IOP measurements with Maklakoff tonometer to clarify the pattern of IOP fluctuations. Patients underwent visual acuity assessment, slit lamp biomicroscopy (HS-5000, Huvitz Co., Seoul, South Korea), gonioscopy with a 3-mirror Goldmann-type lens, static automated perimetry (SAP) using the Humphrey field analyzer (HFA) II 750i (Carl Zeiss Meditec Inc., Dublin, California) 30–2 SITA‐standard test, and SD OCT (Topcon 3D OCT-2000FA Plus; version 7/21/003/0; Topcon GB, Newberry, Berkshire, UK) using 3D Macula, 3D Disc and 3D(V) Glaucoma Analysis in the Macula 3D (V) over a 7.0x7.0-mm scan area. OCT was used to assess thicknesses of the retinal ganglion cell complex (GCC) layers, nerve fiber layer (NFL), ganglion cell and inner plexiform layers (GCL + IPL), and NFL+GCL+IPL; neuroretinal rim (NRR) area, cup-to-disc ratio, optic cup volume and average peripapillary RNFL thickness. All these parameters were automatically calculated by the software. Biomicroscopy and gonioscopy were used to assess iris atrophy; thinning of the pupillary border of the iris; presence of exfoliation material on the pupil margin, anterior lens capsule, and anterior chamber angle; and obliteration of the anterior chamber angle by pseudoexfoliation material. Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK, USA) and EXCEL software. Data are presented as mean and standard deviation (SD). The level of significance p ≤ 0.05 was assumed. Pearson correlation coefficient and coefficient of determination were calculated to assess correlations. Results Mean uncorrected visual acuity (UCVA) was 0.42 ± 0.11 (range, 0.03 to 0.9), and mean best-corrected visual acuity (BCVA), 0.74 ± 0.14. In addition, according to outpatient records, in 25.3% of patients, a mean reduction in visual acuity over the 6-month period was 0.2 ± 0.02 (р < 0.01). In the PEG group, mean intraocular pressure (Pt) was 29.58 ± 1.21 mmHg (range, 21 mmHg to 34 mmHg). In addition, in 11 eyes (28.8%), Pt was increased from 6 AM to 8 AM, and in the rest 27 eyes (71.2%), Pt was normal in the daytime, and significantly increased after 9 PM and before 6 AM. The outpatient examination often failed to identify increased IOP in these patients due to the above fluctuations in IOP. Signs of PXS were identified on slit-lamp biomicroscopy. Particularly, there were grades 1 and 2 iris stroma atrophy in 20 eyes (13.7%) and 17 eyes (11.6%), respectively; thickness of pupillary border of the iris was unchanged in 26 eyes (11%) and reduced in 22 eyes (15%); and exfoliation material was present and absent on the pupil margin in 24 eyes (16.4%) and 14 eyes (9.6%), respectively, and on the anterior lens capsule in 26 eyes (17.8%) and 12 eyes (8.2%), respectively. In addition, exfoliation material was present in the anterior chamber angle in 19 eyes (13.0%) (Fig. 1).
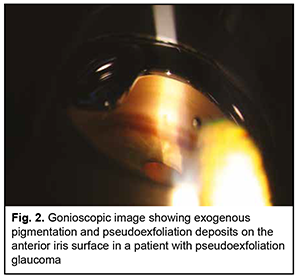
There were gonioscopic changes (Fig. 2) in the anterior chamber angle such as punctate deposits in the iridociliary sulcus in 47.3% of eyes; granular deposits in the anterior chamber angle, in 28.8% of eyes; deposits in the form of conglomerates in 21.2% of eyes; narrow anterior chamber angle in 26.0% of eyes, and completely closed anterior chamber angle in 10.3% of eyes. Increased (grade 3 or 4) anterior chamber angle pigmentation was found in 39.7% of eyes, and Sampaolesi’s line (pigmented line anterior to Schwalbe’s line in the anterior chamber angle), in 15.7% of eyes. High IOP levels ranging from 29 mmHg to 34 mmHg were characteristic for patients with a narrowed or closed anterior chamber angle, increased pigmentation and/or exfoliative conglomerations. Patients with small granular or lace-like exfoliations had an IOP of 22-28 mmHg, and 92% of these patients were referred to the department for cataract surgery with undiagnosed PEG.
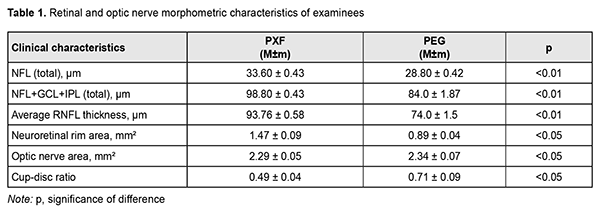
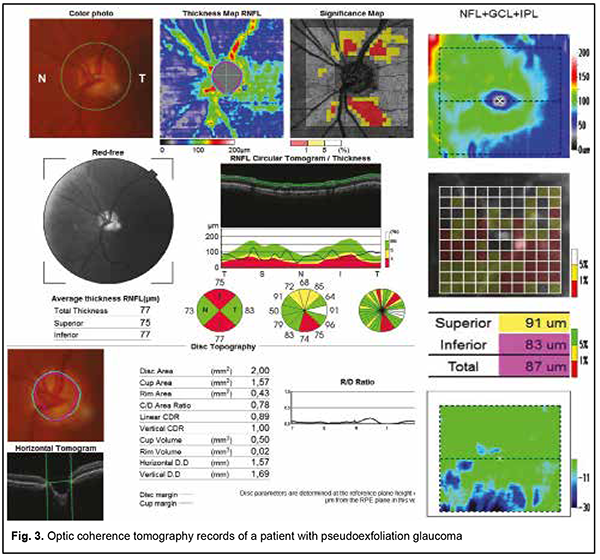
Mydriasis was achieved with mydriatic eye drops only in 63% of eyes, and with a combination of mydriatic eye drops and mydriatic injections in 21.2% of eyes. Mydriasis was not achieved in 15.8% of eyes. Static perimetry showed specific changes in the visual field like a diffuse reduction of MD (48.2% of eyes), expanded blind spot (5.5%), paracentral scotoma (4.0%), nasal step defect (5.5%), focal non-specific defects (13.2%) and concentric visual field narrowing (23.6%). In addition, according to outpatient records, in 37% of patients, mean annual visual field loss was of 20 degrees ± 0.1. Mean MD and pattern standard deviation (PSD) for the PEG group were 5.0 ± 0.78 and 4.50 ± 0.44, respectively, and for the PXS observation group, 2.13 ± 0.18, and 2.03 ± 0.18, respectively. Mean OCT-measured retinal and optic nerve morphometric parameters are presented in Table 1. Example OCT data for a patient with PEG are presented in Fig. 3.
Discussion It has been reported that approximately 87% of patients with PEG had bilateral ocular pseudoexfoliation, and patients with unilateral ocular pseudoexfoliation exhibited clinical changes in the fellow eye over time. In the current study, only 28% of patients had bilateral ocular pseudoexfoliation. In addition, of the eyes with PEG, 11 (28.8%) exhibited a reverse-type 24-hour tonometric curve, whereas 27 (71.2%) showed normal IOP values in the daily hours, and significantly increased IOP values in the evening and early morning hours. Higher IOP values were characteristic for patients with more marked exfoliative or pigment deposits in the anterior chamber angle. These changes attracted attention at once, and most patients with these changes were early diagnosed with glaucoma (mostly, primary open-angle glaucoma). Of the examined patients with signs of hypertension and normal perimetry measurements, only two (4.1%) had retinal and optic nerve morphometric parameters corresponding to mean characteristics of the examined patients without increased IOP. Consequently, these two patients were excluded from the PEG observation group. In 17 patients (11.6%) without perimetric changes, glaucoma (more specifically, early glaucoma) was diagnosed solely by morphometric changes in the GCC. The characteristic features of POAG and PEG have been described in the literature [7] and we took them into account while conducting the analysis of morphometric data. In the current study, OCT showed thinned NFL (33.60 ± 0.43 in eyes with PXS even without increased IOP, with a mean cup-disc ratio of 0.49 ± 0.04, and a significantly thinned NFL of 28.80±0.42 in eyes with PEG, р=0.04) and NFL+GCL+IPL (98.80±0.43 in eyes with PXS and 84.0±1.87 in PEG, р=0.023). Given that the macular retina harbors over half of all ganglion cells, macular OCT study can detect early ganglion cell loss and is a promising parameter of PEG progression. However, the use of this program has not yet become compulsory for all glaucoma suspects. In addition, OCT detected significant RNFL thinning in eyes with PXS, and more RNFL significant in eyes with PEG. This indicated less apparent not only macular but also diffuse peripapillary retinal thinning. Moreover, the RNFL thickness values for eyes with PXS were close to those characterizing the presence of glaucoma process in eyes with open-angle glaucoma, whereas the RNFL thickness values for eyes with PEG corresponded to advanced and severe POAG, which was in agreement with the literature [8]. There was no significant difference in optic disc area and neuroretinal rim area in the current study (р = 0.52). There was a strong direct correlation between changes in GCC (NFL+GCL+IPL) thickness and SAP MD (coefficient of determination R2, 0.77; correlation coefficient r, 0.88). The most prominent thinning was in the central macular area where the ganglion axons are located. Therefore, (1) the reduction in average retinal nerve fiber layer (NFL) thickness and total thinning of GCC layers in patients with pseudoexfoliative glaucoma appeared most indicative for diagnosing PEG, and (2) the presence of pseudoexfoliative syndrome is a factor with the threat of PEG (in the current study, 26% of patients with PXS were found to have PEG). The 24-hour IOP curve was characterized by higher IOP values in the morning hours for 28.8% of eyes with PEG, or the late evening and early morning hours (9 PM to 6 AM), with normal IOP values in the day hours for 71.2% of eyes with PEG. This makes specific monitoring relevant. Detailed retinal NFL thickness monitoring in eyes with PXS may contribute to early identification of eyes at risk for PEG if this thickness is less than 30 μm. Total peripapillary RNFL thinning (mean thickness value, 93.76 ± 0.58 μm) was characteristic for eyes with PXS. Detecting total peripapillary RNFL thickness less than 90 μm demonstrates the development of glaucomatous optic neuropathy, particularly, in eyes with PEG.
References 1.Elhawy E, Kamthan G, Dong CQ, Danias J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum Genomics. 2012 Oct 10;6(1):22. 2.Bezkorovayna IM, Nakonechnyi DO, Jouini Dhia Eddine. [Frequency of occurrence of pseudo-exfoliative glaucoma in patients with pseudo-exfoliative syndrome]. Svit biologii i medytsiny. 2019; 3(69):10–13. 3.Bezditko PA, Melnyk VO, Kolotilov SV. Spectroscopic analysis of intraocular fluid in patients with cataract and pseudoexfoliation syndrome- associated glaucoma. J Ophthalmol (Ukraine). 2019;2:3-6. 4.Plateroti P, Plateroti AM, Abdolrahimzadeh S, Scuderi G. Pseudoexfoliation syndrome and pseudoexfoliation glaucoma: a review of the literature with updates on surgical management. J Ophtalmol. 2015;2015:370371. 5.Kim S, Sung KR, Lee JR, Lee KS. Evaluation of lamina cribrosa in pseudoexfoliation syndrome using spectral-domain optical coherence tomography enhanced depth imaging. Ophthalmology. 2013 Sep;120(9):1798-803. 6.Desai MA, Lee RK. The medical and surgical management of pseudoexfoliation glaucoma. Int Ophthalmol Klin. Fall 2008;48(4):95-113. 7.Gottanka J, Kuhlmann A, Scholz M, Johnson DH, Lütjen-Drecoll E. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4170-81. 8.Vidas S, Popović-Suić S, Novak Lauš K., Jandroković S. et al. Analysis of ganglion cell complex and retinal nerve fiber layer thickness in glaucoma diagnosis. Acta Clin Croat. 2017 Sep;56(3):382-390.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|