J.ophthalmol.(Ukraine).2020;5:62-74.
|
http://doi.org/10.31288/oftalmolzh202056274 Received: 27 July 2020; Published on-line: 27 October 2020 Clinical, morphological, CT and MRI characteristics of anterior skull base and orbital tumors O. I. Palamar,1 E. V. Lukach,2 A.P. Guk, 1 A. P. Maletskyi,3 S. I. Poliakova,3 O. V. Kravets,4 Yu. O. Serezhko,2 D. I. Okonskyi,1 1 Romodanov Neurosurgery Institute, National Academy of Medical Sciences of Ukraine; Kyiv (Ukraine) 2 A.I. Kolomiychenko Institute of Otolaryngology of the National Academy of Medical Sciences of Ukraine; Kyiv (Ukraine) 3 Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) 4 National Cancer Institute, Ministry of Health of Ukraine; Kyiv (Ukraine) E-mail: Erwin@lukach.org TO CITE THIS ARTICLE: Palamar OI, Lukach EV, Guk AP, Maletskyi AP, Poliakova SI, Kravets OV, Serezhko YuO, Okonskyi DI. Clinical, morphological, CT and MRI characteristics of anterior skull base and orbital tumors. J.ophthalmol.(Ukraine).2020;5:62-74. http://doi.org/10.31288/oftalmolzh202056274 Background: Tumors of the anterior skull base and orbit (ASBOT) are more commonly epithelial malignancies arising in the nasal cavity, paranasal sinuses, and orbit, and, of ASBOT, approximately 25%-30% are diagnosed in advanced cases. Primary epithelial tumors of the orbit are located in the lacrimal gland area, and grow invasively with destruction of the roof and external wall of the orbit and intracranial and intracerebral extensions. Purpose: To examine the clinical, morphological, computer tomography (CT) and magnetic resonance imaging (MRI) characteristics of anterior skull base and orbit tumors. Material and Methods: One hundred and ninety-two patients with ASBOT treated at the Kolomiichenko Institute of Otolaryngology and Romodanov Neurosurgery Institute and 110 patients with epithelial lacrimal gland tumors (PELGT) treated at the Filatov Institute were included in the study. Results: A malignant ASBOT was found in 133 patients (69.3%), and a benign, in 59 (30.7%). Most benign cases (84%) were juvenile nasopharyngeal angiofibroma, meningioma or osteoma. The most common primary location of benign ASBOT was extracranial (51% of patients), followed by skull base bone and cartilage (27%) and intracranial (22%). Cancer neoplasms constituted 40% of malignant ASBOT. The primary location of malignant ASBOT was much more commonly extracranial (81% of patients) than skull base bone and cartilage (19%). Of the 110 patients with PELGT, 51 (46.3%) had benign tumors (benign pleomorphic adenoma, 42 (38.2%); mucoepidermoid tumor, 5 (4.5%); myxoma, 3 (2.7%); and oncocytoma, 1 (0.9%)); and 59 (53.7%), malignant tumors (adenocarcinoma, 33 (30.0%); adenoid cystic carcinoma, 18 (16.4%); and malignant pleomorphic adenoma, 8 (7.3%)). Patient complaints were broadly categorized into cerebral, rhynologic, neuro-ophthalmologic and otologic. These complaints were found almost as common in patients with benign as with malignant ASBOT, and were characteristic also for PELGT. Clinical, CT and MRI features for various histomorphological types of benign and malignant neoplasms were described. Conclusion: Large and even giant size is a common feature of ASBOT; this is true also for primary ASBOT diagnosed for the first time during a long asymptomatic stage. We also determined frequencies of injury to various compartments of the intra- and extra-cranial surfaces of the skull base for benign and malignant processes. Benign ASBOT were found to be more commonly located in the lateral skull base, whereas malignant ASBOT were more commonly seen within the floor of the anterior cranial fossa. There are certain grades of intracranial growth for malignant ASBOT and intracranial extension for benign ASBOT, these grades depending mostly on histobiological characteristics and disease duration. Intracranial and intracerebral extensions are the main cause of mortality in patients with PELGT. Keywords: benign and malignant anterior skull-base tumors, epithelial lacrimal gland tumors, clinical features, CT, MRI, diagnostic techniques
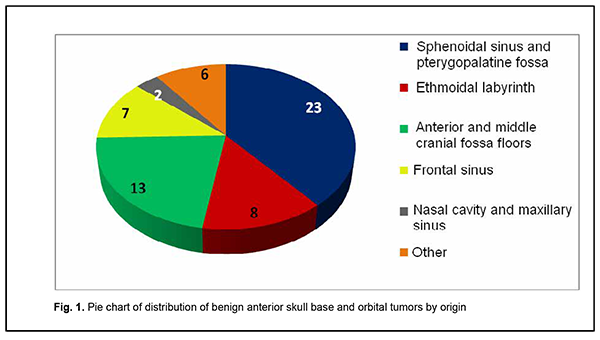
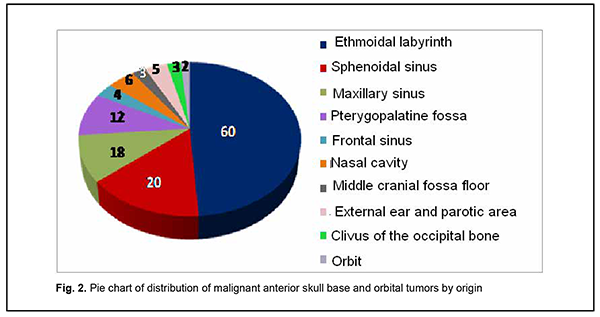
Introduction Tumors of the anterior skull base and orbit (ASBOT) extend intra- and/or extracranially, and invade the face, orbit, nasal cavity, paranasal sinuses, naso- and oropharynx, maxilla, neck, ear, infratemporal and pterygopalatine fossae, and facial bones, with damage to the skull base (anterior and lateral neurocranium and posterior cranial fossa), and penetrate to the intracranial space. Commonly, these are epithelial neoplasms (cancers). They may be cartilaginous or osseous tumors (chordoma, chondrosarcoma, osteosarcoma, osteoblastoma), vascular tumors (angioleiomyoma, hemangiosarcoma, hemangiopericytoma), connective tissue tumors (leiosarcoma, rhabdomyosarcoma, neurofibrosarcoma, neuroblastoma, reticulosarcoma), and neuroepithelial tumors (schwannoma, esthesioneuroblastoma). Juvenile nasopharyngeal angiofibromas (skull base angiofibromas), meningiomas, and intracranial osteomas which may extend extracranially are common among benign ASBOT. The nasal cavity is the most common location for malignant ASBOT (44%), followed by sinus maxillaries (36%), other paranasal sinuses (15%) and orbit (5%) [1]. Approximately 25%-30% of ASBOT are diagnosed in advanced cases. Five-year survival rate for malignant nasal cavity tumors and paranasal sinus tumors with growth into the orbit and/or skull cavity was not more than 16% until 1986, and was increasing thereafter to a value as high as approximately 60% due advances in craniofacial resection. Early morphological diagnosis of the tumor and adequate radical surgical intervention are essential and associated with tumor dissemination within the paranasal sinus region, orbit, skull base, intracerebrally, and into the cavernous sinus [2-5]. Primary epithelial tumors of the orbit are located in the lacrimal gland area (PELGT), and commonly grow invasively with destruction of the roof and external floor of the orbit and intracranial and intracerebral extensions, which is the main cause of mortality [6, 7-12]. Currently, computer tomography (CT) and magnetic resonance imaging (MRI) are the mainstay of preoperative diagnosis of ASBOT and the modalities that enable visualization of the tumor and determination of its anatomic extent, which is crucial for making a decision on treatment strategy. The purpose of the study was to examine the clinical and morphological and intrascopic (CT and MRI) characteristics of anterior skull base and orbit tumors. Material and Methods A total of 192 patients with ASBOT who were treated at the Kolomiichenko Institute of Otolaryngology, Romodanov Neurosurgery Institute were included in the study. Of these, there were 77 women (40.1%) and 115 men (59.9%). Patient age varied from 3 to 72 years, and most were aged either younger than 18 years (43 patients; 22.4%) or 41 to 66 years (77 patients; 40.1%). In addition, 110 patients with primary epithelial lacrimal gland tumors (PELGT) treated at the Filatov Institute were included in the study. Of these, there were 49 male (44.5%) and 61 female (55.5%) patients. Patient age varied from 14 to 75 years (mean ± SD, 45.8 ± 16.6 years). The right orbit was affected in 69 patients (62.7%), and the left orbit in 41 (37.3%). Patients underwent routine general clinical examination, and special examinations (rhinoscopy, endoscopy, CT, MRI, visual acuity, ophthalmoscopy, biomicroscopy, exophthalmometry, perimetry, campimetry, etc.). Biopsy was performed if the tumor was anatomically accessible for biopsy and in case of tumor extension to paranasal sinuses, nasal cavity, sphenoidal sinus, sella turcica, pterygopalatine fossa, and basilar apex. Endonasal (e.g., endoscopic) approach was used for biopsy, and intraoperative navigation was used, if required. However, if the lesion was located at the lacrimal gland, fine-needle aspiration biopsy or incision biopsy was performed. Spiral CT and MRI of the brain, paranasal sinuses, and orbit were administered to determine the tumor origin and extension. The 3-mm sections were used for scanning. Once a tumor was detected, its dimensions were measured, and its density was evaluated. In addition, we determined whether there was bony destruction due to either tumor growth or tumor invasion involving the bone of the skull base. Tumor extension to important skull base structures (e.g., the cavernous sinus and orbital cone) was assessed. Moreover, we assessed cerebral edema and the presence of obstructive hydrocephalus or signs of a dislocated brain stem. Patients underwent MRI imaging at 0.5-1.5 T and 0.5-mm spatial resolution. The MRI helped assess invasion of the adjacent tissues, particularly, dura mater, and dura mater thickening as a reliable sign of intradural tumor growth. Subdural tumor extension with MRI evidence of subarachnoid space deformation and tumor invasion of the cerebral cortex and white matter result in cerebral edema that is reflected by relevant changes on T1- and T2-weighted MRI scans. If there was CT and MRI evidence of orbital injury, we assessed destruction of the orbital medial wall, roof and floor, growth in the periorbit, other orbital structures, orbital muscles and fat, and optic nerve injury. MRI or CT angiography with the use of 3D TOF magnetic resonance angiography (MRA) and magnetic resonance reconstruction (MRR) were performed only in rare cases, enabling to assess the positions of internal and external carotid arteries (ICA and ECA). No contrast medium was required in these cases because the method (MPA) uses blood as an internal contrast medium. Selective angiography of the cerebral vessels was performed using the Seldinger technique. The angiographic apparatus features selectable fields of view (17, 23 and 33 cm) and incorporates fluoroscopy system with fluoroscopy modes of 2.5 to 30 frames per second. The entire research process complied with the ethical standards of the Helsinki declaration and the Council of Europe's Convention on Human Rights and Biomedicine, and the relevant laws of Ukraine. All patients signed informed consent. Statistical analyses were conducted using Statistica 6 (StatSoft, Tulsa, OK, USA) software. Results A malignant ASBOT was found in 133 patients (69.3%), and a benign, in 59 (30.7%). Juvenile nasopharyngeal angiofibroma was the most common benign histological type (22 patients; 37.3%), followed by cartilaginous or osseous tumors (osteoma, fibrous dysplasia; 16 patients; 27.1%), typical, atypical or anaplastic meningioma (12 patients; 20.3%), and sympathetic ganglia or neural tumors (neurofibroma, neurofibromatosis or glomus tumors; 3 patients; 5.1%). In addition, there were 6 cases (10.2%) of hemangioma or pleomorphic adenoma. Therefore, most benign cases (84%) were juvenile nasopharyngeal angiofibroma, meningioma or osteoma. Cell cancer (basal cell carcinoma, transitional cell carcinoma, keratinized or non-keratinized squamous cell carcinoma, anaplastic cell carcinoma, adenoid cystic carcinoma, unspecified malignant neoplasm, or cystadenocarcinoma) was the most common malignant histological type (52 patients; 39.8%) followed by sarcoma (chondrosarcoma, leiomyosarcoma, rhabdomyosarcoma, angiomyosarcoma, lymphosarcoma, polymorphic cell sarcoma or reticular cell sarcoma; 20 patients; 15.0%), cartilaginous or osseous tumors (osteoblastoclastoma, chordoma or chondroma; 16 patients; 12.0%), sympathetic ganglia or neural tumors (malignant neurinoma, paraganglioma; 16 patients; 12.0%), adenocarcinoma (11 patients; 8.3%), esthesioneuroblastoma (9 patients; 6.8%), vascular and lymphoid tumors (hemangiopericytoma, angioleiomyoma, lymphoma, or hemangioendothelioma: 4 patients; 3.0%), and neuroblastoma (neuroblastoma or ganglioneuroblastoma: 4 patients; 3.0%). Of the 110 patients with PELGT, 51 (46.3%) had benign tumors (benign pleomorphic adenoma, 42 (38.2%); mucoepidermoid tumor, 5 (4.5%); myxoma, 3 (2.7%); and oncocytoma, 1 (0.9%)), and 59 (53.7%), malignant tumors (adenocarcinoma, 33 (30.0%); adenoid cystic carcinoma, 18 (16.4%); and malignant pleomorphic adenoma, 8 (7.3%)). Figure 1 shows primary tumor locations for patients with benign ASBOT, and Figure 2, for those with malignant ASBOT.
Benign ASBOT developed somewhat more frequently in the lateral skull base (the middle cranial fossa floor, infratemporal and pterygopalatine fossae) than in the middle skull base (the anterior cranial fossa floor, ethmoid labyrinth, orbit, and sphenoidal sinus), and this difference was mainly due to skull base angiofibroma (Fig. 1). Therefore, the most common primary location of benign ASBOT was extracranial (51% of patients), followed by skull base bone and cartilage (27%) and intracranial (22%). As opposed to benign ASBOT, most malignant ASBOT (60%) developed in the middle skull base (the anterior cranial fossa floor, ethmoid labyrinth, orbit, and sphenoidal sinus) (Fig. 2). The primary location of malignant ASBOT was more commonly extracranial (ethmoid labyrinth; sphenoidal, maxillary and frontal sinuses; nasal cavity, orbit; infratemporal and pterygopalatine fossae, external ear and parotic area; 81% of patients) than skull base bone and cartilage (19%). The intracranial site included the middle cranial fossa floor, anterior cranial fossa floor, and basilar clivus. We found that the process of ASBOT spread may be divided into phases. The phase of bony destruction of the medial orbit wall was followed by that of the anterior cranial fossa as the tissue mass filled the nasal cavity and paranasal sinuses. The bone destruction phase was followed by the epidural expansion of the tumor which was in some cases followed by tumor adhesion to or growth into the dura mater. Tumors of the anterior skull base and orbit are commonly large by the time they are diagnosed due to a prolonged asymptomatic course. The complaints of patients with ASBOT were placed into four broad categories (Table 1).
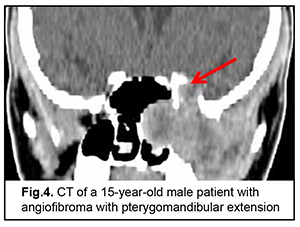
Cerebral and focal neurologic complaints were the most common type. In addition, these complaints were almost as common in patients with benign ASBOT (39.8%) and in those with malignant ASBOT (40.9%) (р > 0.05). Headache, dizziness, fatigue, general weakness, and somnolence were classified as cerebral complaints. Focal neurologic symptoms included facial numbness (impaired CN 5 function) in 15 patients, facial asymmetry (CN 7 dysfunction) in 5 patients, impaired swallowing and phonation (caudal nerve dysfunction) in 3 patients. The severity of cerebral and focal neurologic symptoms depends on the degree of intracranial extension of the lesion and potential cerebral edema. Of note that intracranial hypertension was observed only in patients with significant cerebral edema. Difficulties with nasal breathing, purulent nasal discharge, and epistaxis (due to tumor obstruction of the natural foramina between sinuses and consequently impaired natural ventilation of the nasal cavity and paranasal sinuses) were classified as rhynologic complaints. There was no significant difference in the frequency of these complaints between patients with benign ASBOT (28.7%) and those with malignant ASBOT (27.1%) (р > 0.05). A progradient course of rhynologic symptoms was a feature of our patients with ASBOT. Rhynologic complaints did not improve, but increased in severity with time. It should be noted that, in the current study, epistaxis was occasional and mostly in patients with nasopharyngeal angiofibroma. Reduced visual acuity, diplopia, oculomotor abnormalities, ophthalmoplegia, exophthalmos, tearing, eyelid swelling and chemosis and ocular pain were classified as neuroophthalmologic complaints. Neuroophthalmologic complaints and the third most common type of complaints, and were caused by extension of the craniofacial lesion into the orbit. In addition, these complaints were almost as common in patients with benign ASBOT (21.3%) and in those with malignant ASBOT (25.9%) (р > 0.05). Otologic complaints were the least common in our patients with ASBOT. They included hearing loss, noise, and stuffy ear, and were caused by eustachian tube obstruction and expansive tumor growth or tumor-associated inflammation. Another cause was expansion of the tumor to middle and inner ear structures with their subsequent destruction and hearing loss. Otologic complaints were common in patients with benign ASBOT (10.2%) than in those with malignant ASBOT (6.1%), but the difference was not significant (р > 0.05). It should be noted that the frequency of rhynologic complaints caused by rapid expansion of the tumor in the nasal cavity or orbit and periorbital growth was almost the same as the frequency of rhynologic complaints due to these causes. Patients with benign ASBOT had practically the same complaints as patients with malignant ASBOT, and the severity of these complaints mostly depended on lesion extension and tumor growth duration. Most patients with benign and malignant PELGT had complaints associated with nasal displacement of the globe by the newly formed tissue at the superior and outer surface of the upper eyelid (the lacrimal gland area) and limited upward and outward movement of the globe (53.8% and 58.2% of cases, respectively). In addition, in most patients with benign and malignant PELGT, the tumor mass was dense (92.2% and 96% of cases, respectively), the tumor surface smooth (72.6% and 66.1% of cases, respectively), the tumor mass immobile with respect to the bony orbital walls (66.6% and 83.0% of cases, respectively). The following clinical signs were found significant for differentiating a malignant from a benign PELGT: ptosis (Δ%=15.8, р=0.04), conjunctival hyperemia (Δ%=21.6, р=0.01), diffuse pattern of tumor growth (Δ%=21.5, р=0.05), impossibility of globe reposition (Δ%=22.3, р=0.03), and pain in palpation of the tumor (Δ%=9.4, р=0.04). Benign ASBOT Jjuvenile nasopharyngeal angiofibroma grows slowly, but tends to recur and/or grow into the structures adjacent to the epipharynx and nasal cavity, which may seriously complicate tumor excision. There was CT and MRI evidence that the tumor exhibited sphenoethmoidal and pterygomandibular patterns of growth (Figs 3, 4). An angiofibroma grows in an asymptomatic way extradurally through expanded natural foramina of the middle skull base, particularly, foramen ovale, foramen rotundum and fissura orbitalis superior. The tumor also exhibits extension into the cavernous sinus due to destruction of the lateral walls and floor of the sphhenoidal sinus. Initially, the tumor affects the pterygopalatine and infratemporal fossae, and, subsequently, expands to the cavernous sinus through the natural foramina of the middle skull base, particularly, foramen ovale, that becomes significantly expanded, foramen rotundum and fissura orbitalis superior. In addition, the tumor displaces the cavernous sinus, but becomes located extradurally, beyond the sinus. This is accompanied by symptoms of oculomotor nerve damage, reduced visual acuity due to destruction of the wall of the optic nerve canal and orbital cone, optic nerve compression and tumor mass filling the orbit, which was observed in this case. Patients with angiofibroma usually do not notice such symptoms as moderate headache and stuffy nose. Nasal bloody discharge and oculomotor abnormalities prompted suspicion for a nasal cavity tumor. This required additional examination (particularly, MRI of the brain) that led to finding the tumor.
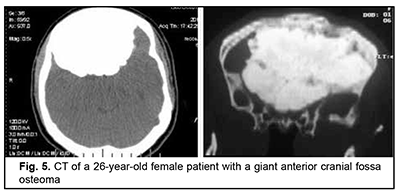
Osteoma originates from osteoblasts of the bony tissue and is categorized as osteoma compactum and osteoma spongiosum. It forms mostly on the external bone surface. When developing in the medullary cavity, it is known as enostosis. The course of the disease is commonly asymptomatic and the growth of osteoma may continue over years, leading to deformation of the facial skeleton and intracranial growth. It is not uncommon that the tumor is found accidentally in the course of examination for mild headache. Fig. 5 shows a giant anterior cranial fossa osteoma that had been growing for decades, with the patient being symptomless for a long time. The tumor extended intracranially, invaded the orbit and nasal cavity, and had a characteristic lumpy surface pattern due to a difference in growth rate between various surface sites. The only symptom noted in the months preceding the patient’s admission to hospital was her psychopathological behavior.
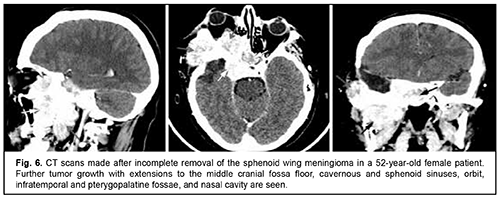
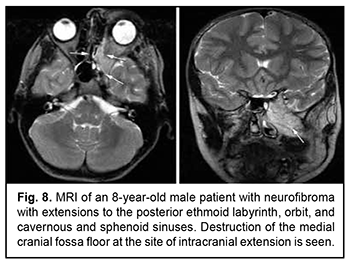
Craniofacial meningioma usually develops due to incompletely removed olfactory fossa meningioma, sphenoid wing meningioma or middle cranial fossa meningioma. A meningioma in any of the above locations, if incompletely removed (particularly, incompletely removed skull base infiltration) may become the origin of the subsequent growth on the inner and outer skull surfaces. We noted intra- and extra-cranial growth of primary meningioma at the ethmoid labyrinth, medial middle cranial fossa and medial posterior cranial fossa. Fig. 6 exemplifies the further spread of the sphenoid wing meningioma to the middle cranial fossa floor, cavernous and sphenoid sinuses, orbit, infratemporal and pterygopalatine fossae, and nasal cavity after incomplete tumor removal. It is noteworthy that, within the field of ophthalmic oncology, sphenoid wing meningiomas constitute a major portion of the large tumors of the anterior skull base and orbit. The tumor extends intracranially and extracranially from the pterygopalatine fossa, leading to destruction of the floor of the medial middle cranial fossa and the involvement of the cavernous sinus (Figs. 7, 8).
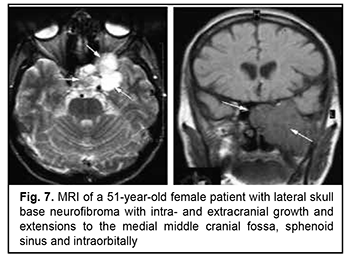
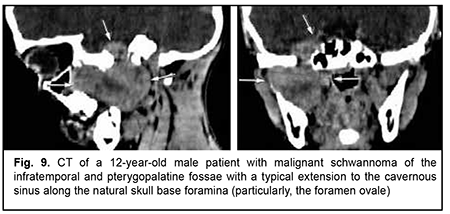
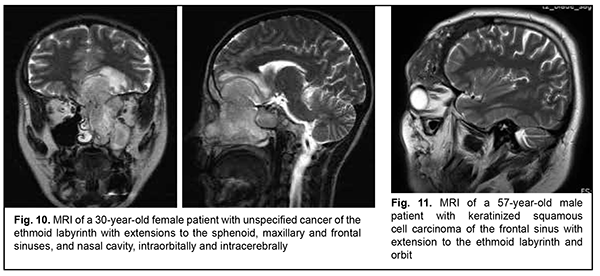
Schwannoma develops from Schwann cells of nerves. Among our patients with schwannoma, the tumor was commonly located within the lateral skull base and extended intracranially through the natural foramina of the skull base. The degree of intracranial extension varied from mild to severe (Fig. 9). Malignant ASBOT An unspecified malignant neoplasm is associated with an extensive invasive growth with significant intracranial and intracerebral extensions, and is accompanied by cerebral swelling and cortical tumor growth. This invasive growth extends also to the orbital tissue, where the tumor grows into the periorbit and orbital fat (Fig. 10).
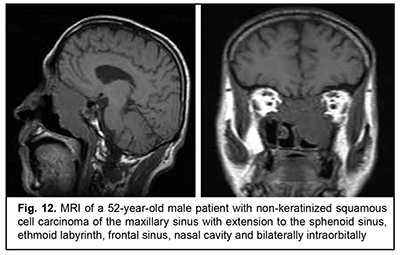
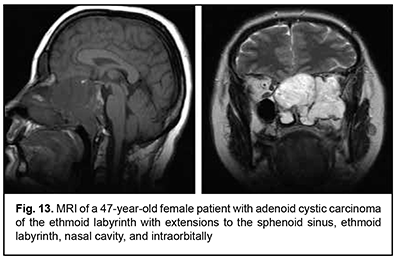
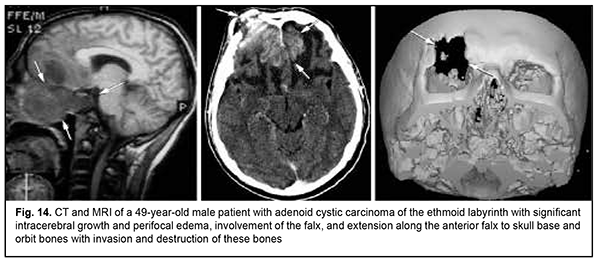
Keratinized squamous cell carcinoma and non-keratinized squamous cell carcinoma are similar in their course and grow locally. Their intracranial growth is mild (Figs 11, 12). Adenoid cystic carcinoma is a highly differentiated cancer that commonly grows locally at the site of origin. The tumors grow expansively (Fig. 13), and may extend intracranially and intracerebrally. Adenocarcinomas are malignant tumors of the lacrimal and salivary glands and paranasal sinuses characterized by early intracerebral invasion with an aggressive course. Fig. 14 shows the adenocarcinoma of the ethmoid labyrinth with significant intracerebral growth and perifocal edema, involvement of the falx, and extension along the anterior falx to skull base and orbit bones with invasion and destruction of these bones.
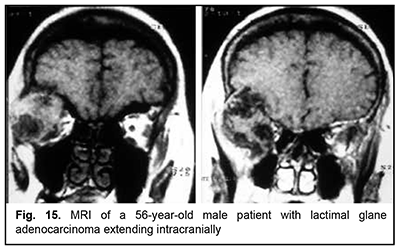
The most aggressive growth and course among epithelial lacrimal gland tumors is seen in adenocarcinomas (Fig. 15). Adenocarcinoma had the highest rate of mortality (67.7%) among PELGT with involvement of the cranial cavity and cerebrum, followed by adenoid cystic carcinoma (47.7%) and pleomorphic adenoma (37.5%). In addition, although pleomorphic adenoma of the lacrimal gland is a benign tumor, its rate of mortality was 9.1%. This could be explained by the fact that, like malignant tumors, these benign tumors tend to recur and transform to adenocarcinomas, adenoid cystic carcinomas and pleomorphic adenomas. The recurrence rate for malignant PELGT was 47.5%, and for benign PELGT, 23.5%. It is noteworthy that we have observed recurrences of pleomorphic adenoma even after 20 years after primary tumor excision.
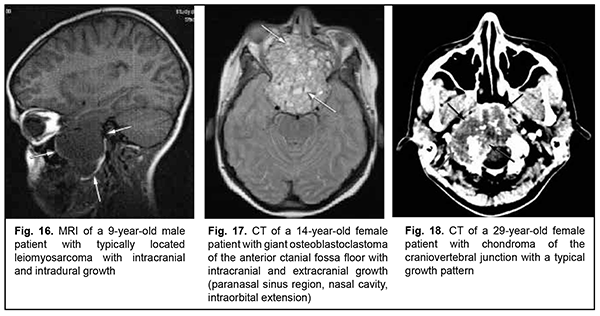
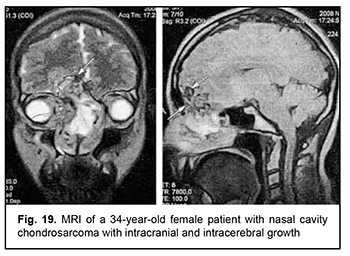
Leiomyosarcoma of the lateral scull base is a tumor with an invasive/ expansive growth pattern, intradural and intracerebral extension, and fusion with surrounding tissues (Fig. 16). Osteoblastoclastoma is a primary bone tumor that may run a benign or malignant course and is characterized by a recurrent growth pattern. Large osteoblastoclastomas frequently bleed and display significant intracranial and extradural extension (Fig. 17). Chordomas develop from remnants of the notochord and can arise at any level of the cranioaxial axis, most commonly, the sphenoidal or sacral level. They have an aggressive and invasive growth pattern with a high risk of recurrence and exhibit early epidural extension. As time goes on, a chordoma (especially, a recurrent chordoma) acquires signs of invasive growth and becomes characterized by intradural and subdural extension. Skull base chordomas are tumors extending centrally, as opposed to skull base chordosarcomas that extend in a lateral direction. Chondroma is a benign tumor that originates from the cartilaginous tissue, shows slow epidural growth and may undergo malignant transformation to chondrosarcoma. In addition, chondroma is characterized by recurrent growth pattern and, with recurrence or after undergoing transformation to chondrosarcoma, it displays invasive growth into the dura mater. The typical location of chondroma is at the craniovertebral junction (Fig. 18). Similar to chondroma, chondrosarcoma runs a course with an expansive growth pattern that is accompanied by bony destruction of the central skull base (sphenoidal sinus, sella turcica, clivus of the occipital bone, and posterior ethmoid labyrinth). However, as opposed to chondroma, chondrosarcoma grows invasively, most commonly extends into the epidural space and frequently recurs, exhibiting intradural or extradural extension. In addition, chondrosarcomas, especially large tumors, frequently fuse with the dura mater (Fig. 19).
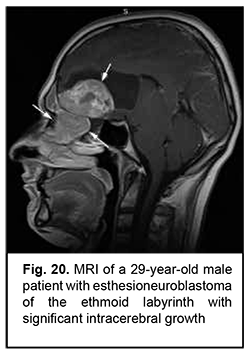
Esthesioneuroblastoma is a tumor that originates from the olfactory epithelium in the superior nasal meatus and grows invasively (Fig. 20). It is located close to the lamina cribrosa, which causes its further intracranial and intracerebral extension. Patients with esthesioneuroblastoma tend to have better survival rates than those with malignant epithelial ASBOT due to radical surgery and effective combination therapy of cancer.
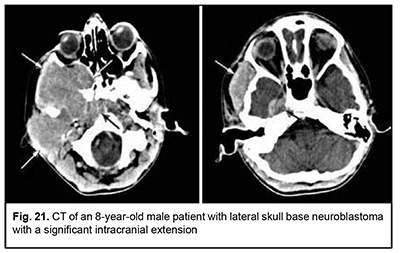
Neuroblastoma originates from neural crest cells of the the sympathetic nervous system, has an aggressive course and invasive growth pattern, and can grow to giant sizes (Fig. 21). Discussion ASBOT arise from the head-and-neck tissues, particularly, (1) bone and cartilage tissues; (2) orbit tissue; (3) facial and hairy scalp skin; (4) remnants of the notochord; (5) nasal cavity mucosa and paranasal sinus mucosa; (6) lining and glandular epithelium; (7) dura mater; and (8) vessels, nerves and sympathetic ganglia [13]. Most ASBOT are the tumors originating in the nasal cavity and paranasal sinuses and extending to and growing into the skull base, and extending intracranially. Nasal cavity and paranasal sinus tumors are commonly malignant [11, 14]. With regard to the origin and extension of benign ASBOT, it has been reported that craniofacial osteomas are tumors with intracranial growth and multidirectional extensions affecting anterior skull base bones [14], whereas meningiomas had primary intra- and extracranial growth at the sites of ethmoid labyrinth, medial middle cranial fossa and medial posterior cranial fossa [14-18], which is in agreement with our findings. Our findings with regard to malignant ASBOT are close to those of others who have reported that adenocarcinomas, unspecified cancers and adenoid cystic carcinomas are malignant tumors characterized by early intracerebral and intracranial invasion with an aggressive course. In addition, these cancers are characterized by continuous invasive growth with extension into the orbit where the tumor grows into the periorbit and orbital fat. These tumors have an unfavourable prognosis for survival [5, 11, 19-24]. The current study demonstrated that an intracranial extension with destruction of the roof and external wall of the orbit is also seen in PELGT that originate in the orbit and exhibit aggressive and recurrent growth [6, 7-10, 12, 25]. The course of cancers of other origins (neuroblastoma, leiomyosarcoma, osteoblastoclastoma, chondrosarcoma) is not less malignant than that of the above cancers. They have an aggressive course and invasive growth pattern, and can grow to giant sizes and extend intradurally and intracerebrally, showing fusion with surrounding tissues [26-31]. According to our findings and those of others, such benign tumors of cartilage origin as chordoma and chondroma grow slowly, show slow and expansive growth, and extend centrally. However, chondroma may undergo malignant transformation to chondrosarcoma and have a recurrent course [11, 19, 29-31]. The expansive growth of these tumors is accompanied by bony destruction with intradural or subdural extensions [28, 31, 32]. Patients with esthesioneuroblastoma tend to have better survival rates than those with other malignant ASBOT if early treated with radical surgery and effective combination therapy [12, 18, 33-36]. Therefore, large and even giant size is a common feature of ASBOT; this is true also for primary tumors diagnosed for the first time. On the basis of the analysis of patient complaints and clinical aspects and comparison of this data with imaging data, we found that ASBOT have a long asymptomatic stage. In addition, we broadly categorized patient complaints into cerebral, rhynologic, neuro-ophthalmologic and otologic, and found that these complaints were almost as common in patients with benign as with malignant ASBOT. The nature of complaints depended on tumor extension to relevant cerebral and skull base structures. We also determined frequencies of injury to various compartments of the intra- and extra-cranial surfaces of the skull base for benign and malignant processes. Compared with malignant ASBOT, benign ASBOT were found to be more commonly located in the lateral skull base (with a rate as high as 40.6%). Malignant ASBOT were more commonly seen within the floor of the anterior cranial fossa (77.2%). There are certain grades of intracranial growth for malignant ASBOT and intracranial extension for benign ASBOT, these grades depending mostly on histobiological characteristics and disease duration. Adenocarcinoma originating in the lacrimal gland is the most aggressive form of epithelial cancer of the orbit with a rate of mortality of 67.7%. While growing, it causes destruction of the roof and external wall of the orbit with extension into the temporal fossa and cranial cavity and involvement of the cerebrum.
References 1.Polishchuk MIe, Lukach EV, Opanaschenko GO. [Combined ethmoidectomy (external transnasal and transcranial frontal) for the malignant tumor of the ethmoid labyrinth]. Zhurnal vushnykh, nosovykh i gorlovyjh khvorob. 1995; 3:43-5. Ukrainian. 2.Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study. Ganly I, Patel SG, Singh B, et al. Head Neck. 2005 Jun;27(6):445-51. 3.Daele JJ, Vander Poorten V, Rombaux P, Hamoir M. Cancer of the nasal vestibule, nasal cavity and paranasal sinuses. B-ENT. 2005;Suppl 1:87-94; quiz 95-6. 4.Origitano TC, Petruzzelli GJ, Leonetti JP, Vandevender D. Combined anterior and anterolateral approaches to the cranial base: complication analysis, avoid¬ance, and management. Neurosurgery. 2006 Apr;58(4 Suppl 2):ONS-327-36; discussion ONS-336-7. 5.Taghi A, Ali A, Clarke P. Craniofacial resection and its role in the management of sinonasal malignancies. Expert Rev Anticancer Ther. 2012 Sep;12(9):1169-76. 6.Brovkina AF. [Diseases of the orbit: a guide for physicians]. 2nd ed. Moscow: Meditsinskoie informatsionnoie agenstvo; 2008. Russian. 7.Poliakova SI. [Epithelial lacrimal gland tumors: clinical features, prognosis and treatment]. Abstract of the Dissertation for the Degree of Dr Sc (Med). Odesa: Filatov Institute of Eye Diseases; 2011. Ukrainian. 8.Lin SC, Kau HC, Yang CF, et al. Adenoid cystic carcinoma arising in the inferior orbit without evidence of lacrimal gland involvement. Ophthalmic Plast Reconstr Surg. Jan-Feb 2008;24(1):74-6. 9.Vagefi MR, Hong JE, Zwick ОM, et al. Atypical presentations of pleomorphic adenoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg. Jul-Aug 2007;23(4):272-4. 10.Bernardini FP, Davoto MH, Croxatho JO. Epithelial tumors of the lacrimal gland: an update. Curr Opin Ophthalmol. 2008 Sep;19(5):409-13. 11.Nicolai P, Redaelli de Zinis LO, Facchetti F, et al. Craniofacial resection for vascular leiomyoma of the nasal cavity. Am J Otolaryngol. Sep-Oct 1996;17(5):340-4. 12.Demiroz C, Gutfeld O, Aboziada M, et al. Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys. 2011 Nov 15;81(4):e255-61. 13.Maroldi R, Ambrosi C, Farina D. Metastatic disease of the brain: extra-axial metastases (skull, dura, leptomeningeal) andtumour spread. Eur Radiol. 2005 Mar;15(3):617-26. 14.Ichimura K. Analysis of tumor recurrence following anterior skull base surgery. Eur Arch Otorhino¬laryngol. 1998;255(3):155-62. 15.Park MC, Goldman MA, Donahue JE, et al. Endonasal ethmoidectomy and bifrontal craniotomy with craniofacial approach for resection of frontoethmoidal osteoma causing tension pneumocephalus. Skull Base. 2008 Jan;18(1):67-72. 16.Henderson JW. Orbital Tumors. 3rd ed. New York: Raven Press; 1994. 17.Kuriakose MA, Trivedi NP, Kekatpure V. Anterior skull base surgery. Indian J Surg Oncol. 2010 Apr;1(2):133-45. 18.Weber SM, Kim J, Delashaw JB, Wax MK. Radial forearm free tissue transfer in the management of persistent cerebrospinal fluid leaks. Laryngoscope. 2005 Jun;115(6):968-72. 19.Shah JP, Kraus DH, Bilsky MH, et al. Craniofacial Resection for Malignant Tumors Involving the Anterior Skull Base. Arch Otolaryngol Head Neck Surg. 1997 Dec;123(12):1312-7. 20.Patel SG, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003 Sep 15;98(6):1179-87. 21.Elloumi F, Boujelbene N, Ghorbal L, et al. Esthesioneuroblastoma. Bull Cancer. 2012 Dec;99(12):1197-207. 22.Teknos TN, Smith JC, Day TA, et al. Microvascular free tissue transfer in reconstructing skull base defects: lessons learned. Laryngoscope. 23.Tsai EC, Santoreneos S, Rutka JT. Tumors of the skull base in children: review of tumor types and management strategies. Neurosurg Focus. 2002 May 15;12(5):e1. 24.Currie ZL, Rose G E. Long-term risk of recurrence after intact excision of pleomorphic adenomas of the lacrimal gland. Arch Ophthalmol. 2007 Dec;125(12):1643-6. 25.Blitzer A, Post KD, Conley J. Craniofacial resection of ossifying fibromas and osteomas of the sinuses. Arch Otolaryngol Head Neck Surg. 1989 Sep;115(9):1112-5. 26.Carrabba GA, Dehdashti R, Gentili F. Surgery for clival lesions: open resection versus the expanded endoscopic endonasal approach. Neurosurg Focus. 2008;25(6):E7. 27.Cheesman AD, Lund VJ, Howard DJ. Craniofacial resection for tumors of the na¬sal cavity and paranasal sinuses. Head Neck Surg. Jul-Aug 1986;8(6):429-35. 28.Emery E, Alaywan M, Sindou M. [Respective indications of orbital and/or zygomatic arch removal combined with fronto-pteriono-temporal approaches. 58 cases]. Neurochirurgie. 1994;40(6):337-47. French. 29.Falcioni M, Taibah A, De Donato G, et al. [Lateral approaches to the clivus]. Acta Otorhinolaryngol Ital. 1997 Dec;17(6 Suppl 57):3-16. Italian. 30.Uttley D. Transfacial approaches to the skull base. Adv Tech Stand Neurosurg. 1997;23:145-88. 31.Sartoris A, Cortesina G, Busca GR. [Anterior craniofacial resection in the treatment of malignant nasal-paranasal sinus tumors with intracranial extension]. Acta Otorhinolaryngol Ital. 1991;11(3):317-27. 32.van Buren JM, Ommaya AK, Ketcham AS. M. Ten years’ experience with radical com¬bined craniofacial resection of malignant tumors of the paranasal sinuses. J Neurosurg. 1968 Apr;28(4):341-50. 33.Cantrell RW, Ghorayeb ВY, Fitz-Hugh GS. Esthesioneuroblastoma: diagnosis and treat¬ment. Ann Otol Rhinol Laryngol. Nov-Dec 1977;86(6 Pt 1):760-5. 34.Simal Julián JA, Miranda Lloret P, Cárdenas Ruiz-Valdepeñas E, et al. Esthesioneuroblastoma. Transcribiform-transfovea ethmoidalis endonasal expanded approach. Technical note. Neurocirugia (Astur). 2012 Jul;23(4):157-63. 35.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007 Mar;133(3):276-80. 36.Pendleton C, Raza SM, Boahene KD, Quinones-Hinojosa A. Transfacial approaches to the skull base: the early contributions of Harvey Cushing. Skull Base. 2011 Jul;21(4):207-14.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|