J.ophthalmol.(Ukraine).2020;4:50-55.
|
http://doi.org/10.31288/oftalmolzh202045055 Received: 24 April 2020; Published on-line: 27 August 2020 Ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan N.F. Bobrova, T.A. Sorochynska, N.I. Molachaniuk, A.Iu. Bratishko SI "Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: filatov.detskoe7@gmail.com TO CITE THIS ARTICLE: Bobrova NF, Sorochynska TA, Molachaniuk NI, Bratishko AIu. Ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan. J.ophthalmol.(Ukraine).2020;4:50-55. http://doi.org/10.31288/oftalmolzh202045055 Background: Intravitreal chemotherapy (IVC) has become a common eye-preserving treatment for retinoblastoma (RB). However, there are still unsolved issues with regard to dosage of the cytostatic agent, its ultrastructural effects on healthy retinal cells, and late complications of the therapy. Purpose: To identify and assess ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan. Material and Methods: Six eyes of three Chinchilla rabbits (age, 5–6 months; weight, 2.5–3 kg) underwent clinical and electron miscroscopy examination. Both eyes of animals ##1, 2 and 3 received a single dose of intravitreal melphalan of 5, 10 and 20 ?g, respectively. Intravitreal injection was performed in a standard manner. The eyes were enucleated 4 weeks after injection. After enucleation, material for histology was taken in a routine manner and examined in a PEM-100-01 electron microscope. Results: Intravitreal injection of various doses of melphalan had no general toxic effect on the animals. No retinal hemorrhage, vitreous hemorrhage, retinal tear or detachment was observed at all follow-up points, regardless of how large an intravitreal dose of melphalan was administered. No complications were observed postoperatively. Throughout a follow-up period of 4 weeks, there were no focal fundus changes in the eyes that received a single intravitreal injection of 5 ?g melphalan. In the eyes that received a single intravitreal injection of 10-?g or 20-?g melphalan, we noted a range of focal fundus changes from as mild as a few small isolated sites of slight depigmentation to as severe as pigment redistribution with the emergence of brighter and greater depigmentation regions in the retina. Ultrastructural changes corresponded to ophthalmoscopic changes. Electron microscopy revealed destructive changes in the retinal pigment epithelium (RPE), photoreceptors, and M?ller cells of the rabbit retina. The minimum structural changes in the RPE and photoreceptor cells were seen after an injection of the smallest dose (5 ?g melphalan). After a 10-?g or 20-?g one-time dose of intravitreal melphalan, we observed severe changes (such as cell swelling, hypertrophy, destruction and disintegration) in retinal cells, disorganized retinal layers with loss of typical ultrastructural features, gliosis and necrosis. Conclusion: On the basis of our findings, we can conclude that intravitreal melphalan is relatively safe when administered as a one-time 5-?g injection, and minimally toxic when administered as a one-time 10-?g injection. However, when administered as a one-time 20-?g injection, it resulted in severe changes such as gliosis and necrosis in retinal cells. Keywords: intravitreal chemotherapy, melphalan, electron microscopy, retina, experiment
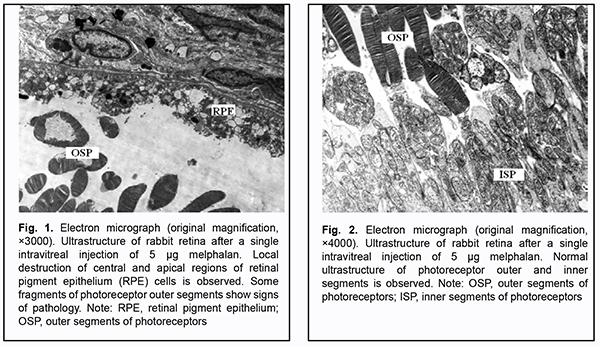
Introduction Eye preserving therapy has become a major approach to treating retinoblastoma (RB) in the two most recent decades. Targeted delivery of anticancer agents to RB cells in the form of intra-arterial, subconjunctival, subtenon and intravitreal chemotherapy (CHT) has become widely used. Intraocular drug delivery is the only mode that currently directly broaches the blood retinal barrier and thereby attains the highest peak intravitreal or intraretinal drug concentrations [1]. Erison and Rosengren first investigated the use of intravitreal chemotherapy for RB in the early 1960s [2], but reports of extraocular tumor spread limited the use of this treatment modality. In 1987, Inomata and Kaneko [3] from Japan found L-phenylalanine mustard (melphalan) to be the most sensitive chemotherapeutic agent against RB based on testing of 12 agents, and a dose of 4 ?g/ml achieved complete tumor suppression. Melphalan is a cytostatic that alkylates intracellular molecular structures, including DNA and RNA, and acts by causing modification and cross-linking of DNA, thus inhibiting tumor or lymphoid cell division and causing cell depth. It induces primary damage to DNA macromolecules, impairs DNA polymerization and replication, promotes production of defective DNA and RNA forms, and inhibits protein synthesis. Melphalan induces DNA inter-strand cross-linkages, resulting in cytotoxicity against both dividing and non-dividing tumor cells and preventing cell replication. Impairment or blocking of mitotic activity is an early sign of damage to tumor cells. In addition, melphalan and products of its biotrasformation are involved in tumor cell metabolism. The earliest ultramicroscopic changes can be found in mitochondria. When total tumor cell death occurs, vital organs (excluding the marrow) do not exhibit irreversible changes, whereas stimulation of proliferation if the connective tissue surrounding the tumor and regeneration of nerve fibers are a characteristic morphological feature. In Ukraine, melphalan is registered as an anti-cancer agent under the trade name of Alkeran, and is recommended for treating late neuroblastoma in children. Intravitreal CHT against retinoblastoma cells was revived in 2003 by Kaneko and Suzuki [4] from Japan and is considered the most promising treatment for the disease. However, there are still unsolved issues with regard to dosage of the cytostatic agent, its ultrastructural effects on healthy retinal cells, and late complications of the therapy. The purpose of the study was to identify and assess ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan. Material and Methods Six eyes of three Chinchilla rabbits (age, 5–6 months; weight, 2.5–3 kg) underwent clinical and electron microscopy examination. The animals were housed under standard vivarium conditions. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Both eyes of animals ##1, 2 and 3 received a single dose of intravitreal melphalan of 5, 10 and 20 ?g, respectively. Intravitreal injection was performed in a standard manner as follows. First, topical anesthetic was instilled into the conjunctival sac. Second, the operative field was cleansed with 0.5% chlorhexidine in alcohol. Third, a blepharostat was used to open the eyelids. Fourth, the intended injection site was marked 4 mm posterior to the limbus in the superonasal quadrant. Fifth, the conjunctiva and sclera were pierced with a 30-G insulin syringe needle to allow slow intravitreal injection of 0.1 mL of the melphalan (Alkeran) solution prepared ex tempore. After each intravitreal injection of melphalan, animals received topical disinfectants and antibacterial therapy. Animals were followed up at days 1, 3, and 7, and each week thereafter. General well being was assessed. In addition, we assessed inflammatory response of the anterior eye to intravitreal injection of melphalan and the transparency of the optic media. Direct ophthalmoscopy was performed under full mydriasis. Ultrastructural studies of the retina were performed 4 weeks after intravitreal injection of melphalan. Rabbits were euthanized by air embolism. After the rabbits were euthanized by air embolism, eyes were enucleated and fragments of posterior eye tissue were fixed using 2.5% glutaraldchyde in 7.4 pH phosphate-buffered saline, post-fixed with 1% osmic axid in 7.4 pH phosphate-buffered saline. Sections were dehydrated with ascending grades of alcohol and embedded in Epon-araldite mixture. Ultrathin sections were cut, stained with Reynolds’ (1963) lead citrate [5], and examined in a PEM-100-01 electron microscope. Results and Discussion There was no perioperative complication regardless of how large an intravitreal dose of melphalan was administered. The general health levels of the rabbits were satisfactory, and no general toxic effect of melphalan on the animals was noted throughout follow-up. In all rabbits, local conjunctival hyperemia was noted inferiorly at the site of melphalan injection the next day, and significantly subsided and disappeared 3 days and 7 days, respectively, after intervention. At all follow-up assessments, the anterior segment was normal, the media were transparent, and no hemorrhage, inflammatory response, infectious or other complications was observed. Fundus changes and ultrasrtructural findings varied depending on the dose of melphalan administered. Throughout a follow-up period of 4 weeks, there were no focal fundus changes in the eyes that received a single intravitreal injection of 5 ?g melphalan. Four weeks after surgery, electron microscopy found various destructive changes at some retinal pigment epithelium (RPE) locations. There were sites with better preserved RPE cells. The ultrastructure of photoreceptor cells in such foci appeared rather well preserved (Fig. 2), and that of cells in other retinal compartments appeared normal.
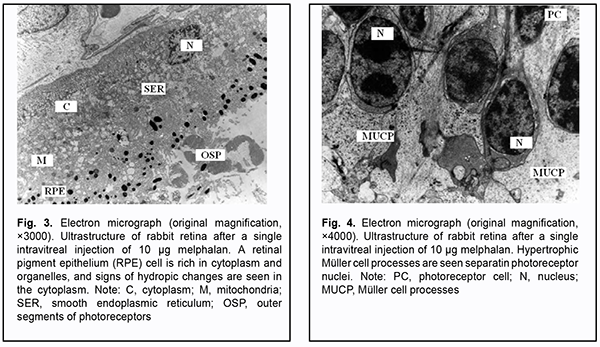
In rabbit #2 (the eyes that received a single intravitreal injection of 10 ?g melphalan), ophthalmoscopic changes in the form of a few small isolated sites of slight depigmentation were infrequently seen. In the rabbit retina, sites with almost normal structure alternated with sites where cells appeared to be damaged and sometimes destroyed. RPE cells were rich in cytoplasm and organelles, and hydropic changes were apparent in the cytoplasm. Membrane disks in the outer segments of photoreceptors appeared to be destroyed, and photoreceptors showed swelling of the inner and outer segments (Fig. 3). Photoreceptor cell (PHC) nuclei were surrounded by hypertrophic M?ller cell (MUC) processes (Fig. 4). Cytoplasmic elements of M?ller cells appeared to be destroyed at some sites. Some ultrastructural elements of the retinal photoreceptor layer and retinal inner layer appeared. Processes of M?ller cells at the inner limiting membrane (ILM) also appeared hypertrophic.
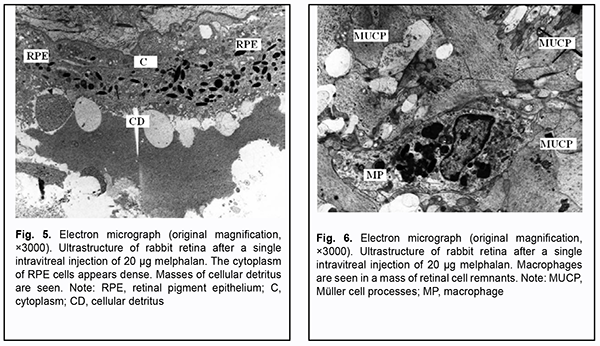
In rabbit #3 (the eyes that received a single intravitreal injection of 20 ?g melphalan), fundus changes were characterized by pigment redistribution and the emergence of brighter and greater depigmentation regions as early as week 1, with an insignificant increase in depigmentation by the end of week 2 after injection. No retinal hemorrhage, vitreous hemorrhage, retinal tear or detachment was observed at all follow-up points, regardless of how large an intravitreal dose of melphalan was administered. After a single intravitreal injection of 20 ?g melphalan, some retinal structures appeared to be destroyed or showed apparent disorder. The cytoplasm of RPE cells was dense and rich in small mitochondria, and apical structures mostly appeared destroyed. Masses of structureless material and cellular detritus were observed subendothelially (Fig. 5). Retinal layers appeared disorganized. Retinal photoreceptor cells and neurons practically lost their typical ultrastructural features. Consequently, it is impossible to determine what class belongs to such a cell with lost ultrastructural features. Affected sites with less damage to retinal elements were occasionally seen. There was an apparent gliosis reaction of M?ller cells with destruction of intracellular structures or necrosis. Macrophages were occasionally present in a mass of cell remnants (Fig. 6). Structures of the RPE appeared partially preserved, and some sites of relatively preserved retinal cells were seen; this is likely because by that time, 4 weeks had passed after a single intravitreal injection of 20 ?g melphalan, and ultrastructure of some cells may be restored.
To the best of our knowledge, there have been scarce reports of intravitreal injection of various doses of melphalan in animals. Ueda and colleagues [6] studied effects of an intravitreal injection of melphalan on the electroretinogram and on the retinal structure in albino rabbits to establish the non-toxic dose for its intravitreal use. The a-wave, the b-wave, the c-wave, the oscillatory potential and the retinal structure remained unchanged after 10-?g injection but greatly deteriorated after 90-?g injection. A 10-?g melphalan injection is equal to an intravitreal concentration of about 5.9 ?g/ml if evenly diffused in the rabbit vitreous. In addition, it corresponds to an intravitreal injection of 20-30-?g melphalan in humans. Considering that colony formation of in vitro retinoblastoma cells is completely suppressed by melphalan at 4 ?g/ml concentration, Ueda and colleagues concluded that an intravitreal application of melphalan could be used as a non-surgical treatment for retinoblastoma. Shimoda and colleagues [7] investigated the toxic effects of perfusion of intravitreal melphalan at concentrations of 5-, 10-, and 20- ?g/ml during vitrectomy on the rabbit retina. In the 5-microg/ml perfusion group, the ERGs and histology showed no substantial changes compared with control fellow eyes during 28 days postoperatively. In the 10- and 20-microg/ml groups, the mean a-wave amplitude decreased to 52% and 31% respectively of the fellow eye; the mean b-wave amplitude decreased to 52% and 19% respectively. Histologic sections showed necrosis of the inner nuclear layer and thinning of the outer nuclear layer in the 10-?g/ml group. Loss of the outer nuclear layer and the photoreceptor layer and necrosis of the inner nuclear layer were observed in the 20-?g/ml group. Shimoda and colleagues concluded that the intravitreal 5- ?g/ml melphalan perfusion during vitrectomy appeared to be nontoxic to the retina, and the therapeutic modality might be a potential treatment for RB with vitreous seeding. However, a judgement with regard to total dose of melphalan introduced intravitreally cannot be made on the basis of findings of those studies, because information on perfusion duration was not provided by the authors, and, consequently, it is unknown which dose is safe and does not cause histomorphological changes in the rabbit retina when used as a single or multiple dose [7]. Shah and colleagues [8] used a 1-?g melphalan injection in a murine retinoblastoma model. One week after intravitreal melphalan therapy, tumor burden was significantly reduced by 85% compared to control; three weeks after treatment with a single injection, tumor burden remained significantly reduced by 83%. Intravitreal melphalan was shown to significantly reduce the density of new vessels despite no significant efect on total vasculature. Hypoxia was shown to transiently decrease 1 week after injection with this effect diminishing by 3 weeks. No significant toxicities were seen on histopathologic examination following intravitreal melphalan [8]. Cassoux and colleagues [9] showed complete tumor regression in 2/6 mice 15 days following subretinal injection of melphalan (500 ?g/kg). However, a judgement with regard to ultrastructural retinal changes after a one-time intravitreal injection of melphalan cannot be made on the basis of findings of those studies. In the current study, rabbit eyes treated with a one-time intravitreal injection of 5 ?g melphalan showed only mild focal changes in RPE cells and photoreceptor outer segments, which is in agreement with findings by Shimoda and colleagues [7]. However, our ultrastructural study of rabbit eyes treated with a one-time intravitreal injection of 10 ?g melphalan showed destructive changes that were more severe in RPE cells, inner and outer segments of photoreceptor cells, and M?ller cells of the retina. In addition, a one-time intravitreal injection of 20 ?g melphalan resulted in severe changes in above structures of the retina, leading to retinal gliosis and necrosis. This is in accord with findings by Shimoda and colleagues [7] for intraocular melphalan perfusion at concentrations of 10 and 20-?g/ml during vitrectomy in rabbits. These findings, however, disagree with those by Ueda and colleagues [6] who noted mild changes in the retina of rabbit eyes treated with an intravitreal injection of 20 ?g melphalan. Our study demonstrated that the extent of clinical and ultrastructural changes in the retina depends on the dose of the agent injected. Slight structural changes in the RPE and photoreceptor cells were seen even after injection of the smallest dose (5 ?g melphalan). The severity and extent of damage to all retinal layers increased with an increase in the injected dose of the cytostatic. The focal pattern of ultrastructural changes (sites with almost normal structure alternated with sites where cells appeared to be damaged and sometimes destroyed) is worthy of clinical attention. Conclusion Intravitreal injection of various doses of melphalan had no general toxic effect on the animals and resulted in no changes in either anterior segment of the eye or optic media. The major toxic effect was exerted on the retina. Clinical and ultrastructural changes in the retina depended on the dose of the agent injected. Specifically, changes were absent after injection of the smallest dose (5 ?g melphalan). In addition, after a one-time injection of 10 ?g melphalan, there were mild ophthalmoscopic changes (a few small isolated sites of slight depigmentation) and mild ultrastructural changes (destructive changes at some retinal RPE locations and in the layer of the photoreceptor outer segments). Moreover, after a one-time injection of 20 ?g melphalan, we observed retinal changes in the form of sites of pigment redistribution and depigmentation, and ultrastructural changes presenting as disorganized retinal layers with abnormal ultrastructure of retinal photoreceptors and neurons, and M?ller cell gliosis with destruction of intracellular structures or necrosis. On the basis of our findings, we can conclude that intravitreal melphalan is relatively safe when administered as a one-time 5-?g injection, and minimally toxic when administered as a one-time 10-?g injection. However, when administered as a one-time 20-?g injection, it resulted in severe changes such as gliosis and necrosis in retinal cells. References 1.Edelhauser HF, Rowe-Rendleman C, Robinson M, et al. Ophthalmic Drug Delivery Systems for the Treatment of Retinal Diseases: Basic Research to Clinical Applications. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5403-20. 2.Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh). 1961;39:569-76. 3.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78:858–68. 4.Kaneko A, Suzuki S. Eye-Preservation Treatment of Retinoblastoma with Vitreous Seeding. Jpn J Clin Oncol. 2003 Dec;33(12):601-7. 5.Reуnolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 1963 Apr;17(1):208-12. doi: 10.1083/jcb.17.1.208. 6.Ueda M, Tanabe J, Inomata M, et al. [Study on conservative treatment of retinoblastoma - effect of intravitreal injection of melphalan on the rabbit retina]. Nihon Ganka Gakkai Zasshi. 1995 Nov;99(11):1230-5. 7.Shimoda Y, Hamano R, Ishihara K, et al. Effects of intraocular irrigation with melphalan on rabbit retinas during vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008 Apr;246(4):501-8. 8.Shah N, Pham D, Murray T, et al. Intravitreal and Subconjunctival Melphalan for Retinoblastoma in Transgenic Mice. J Ophthalmol. 2014;2014:829879. 9.Cassoux N, Assayag F, Chouchane-Mlik O, et al. Intraocular treatments of a new orthotopic primary human retinoblastoma xenograft. Invest Ophthalmol Vis Sci. March 2012;53(3):6869. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|