J.ophthalmol.(Ukraine).2020;4:45-49.
|
http://doi.org/10.31288/oftalmolzh202044549 Received: 15 June 2020; Published on-line: 27 August 2020 Histomorphology of the healthy fellow eye in the rabbit with unilaterally induced non-infectious anterior and intermediate uveitis O. Dorokhova, E. Maltsev, O. Zborovska, Meng Guanjun SI "Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: dorochovaa@gmail.com TO CITE THIS ARTICLE: Dorokhova OE, Maltsev IeV, Zborovska OV, Meng Guanjun. Histomorphology of the healthy fellow eye in the rabbit with unilaterally induced non-infectious anterior and intermediate uveitis. J.ophthalmol.(Ukraine).2020;4:45-49. http://doi.org/10.31288/oftalmolzh202044549 Background: It is important to use objective methods for assessing intraocular inflammation in the practical management of uveitis. Previously, we have found that, on day 1 of unilaterally induced non-infectious anterior and intermediate uveitis in rabbits, the temperature in ciliary body projection onto the ocular surface increased to 35.7°С (р = 0.002) for the challenged eyes and to 35.0°С (р=0.05) for the fellow eyes compared to eyes of intact rabbits (34.1°С). Purpose: To assess the histomorphology of the healthy fellow eye in rabbits with unilaterally induced non-infectious anterior and intermediate uveitis. Material and Methods: Seventeen Chinchilla rabbits (34 eyes; weight, 2.5-3.0 kg) were included in this study. Non-infectious anterior or intermediate uveitis was induced in the right eye of each rabbit, while the left was intact. Histomorphological studies of the fellow eyes were performed at various time points after challenge. Results: The fellow eyes showed normal histomorphology. Conclusion: A significant increase in the temperature in ciliary body projection onto the ocular surface to 35.0°С (р=0.05) for the fellow eyes compared to eyes of intact rabbits on day 1 of induced non-infectious anterior and intermediate uveitis in rabbits was not accompanied by any histomorphological or pathological changes, and was functional in nature. Keywords: non-infectious anterior uveitis, non-infectious intermediate uveitis, ocular surface temperature, histomorphological status
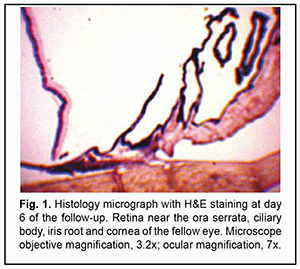
Introduction Although uveitis can be classified as a rare (orphan) disease, it is the fourth most common cause of visual disability in developing countries [1-5]. It is important to use objective methods for assessing intraocular inflammation in the practical management of uveitis. Unfortunately, today laser flare photometry is the only available objective method of determining the activity and degree of inflammation. The laser flare meter has been introduced for quantification of anterior chamber protein and cells, which allows detecting even subclinical inflammation. The technique is employed both for disease diagnosis and monitoring [6-10]. Laser flare photometry is most useful in anterior uveitis. However, in intermediate uveitis, this tool is less reliable than in anterior uveitis and has certain limitations. In addition, cataract, corneal opacities, pupil size, intraocular lens and shallow anterior chamber can affect the results of laser flare-cell photometry [11]. We have given attention to studies on local body temperature responses as a potential alternative method for quantitative assessment of intraocular inflammation. Studies on the basis of local body temperature measurements have been already widely used in various medical specialities like neurology, vertebrology, orthopedics, oncology, phlebology, gastroenterology, endocrinology, sports medicine, traumatology, heart surgery, otorhinolaryngology, dentistry, etc. [12-25]. We believe it is reasonable to conduct a preclinical animal study on local body temperature measurements in uveitis, in an attempt to compare temperature data not only with the clinical picture but also with the results of morphological studies reflecting the actual status of intraocular inflammation. Previously, we have found that the pattern of heat exchange in intact rabbit’s ocular surface (temperature of the ocular surface in the projection of the ciliary body is influenced by autonomic thermoregulation and is relatively stable at small environmental temperature variations) allows for modeling of unilateral ocular pathological processes that would have a change in local body temperature in the projection of the ciliary body as an objective marker [26]. On day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, the temperature in ciliary body projection onto the ocular surface increased to 35.7°С (р = 0.002) for the challenged eyes and to 35.0°С (р=0.05) for the fellow eyes compared to eyes of intact rabbits, which was likely to be caused by the response of the autonomic nervous system in the presence of initiation of inflammatory process. On day 5, a less significant difference appeared in the ocular surface temperature between the challenged eyes and fellow eyes, which was likely to be caused by decreased temperature response of the autonomic nervous system as well as by a gradual decrease in temperature of the fellow eye [27]. Consequently, we decided to conduct a histological study of the healthy fellow eye in these animals to definitely rule out sympathetic ophthalmia as a possible cause of such an increase in temperature. Therefore, the purpose of this study was to assess the histomorphology of the healthy fellow eye in rabbits with unilaterally induced non-infectious anterior and intermediate uveitis. Material and Methods Seventeen Chinchilla rabbits (34 eyes; weight, 2.5-3.0 kg) were included in this study. They were divided into two experimental series, series 1 (7 rabbits) and series 2 (10 rabbits). Animals were used in the experiments after being quarantined for 2 weeks. They were housed at the normal vivarium temperature of 18 до 25°С, and fed and watered conventionally. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Non-infectious anterior or intermediate uveitis was induced in the right eye of each rabbit, while the left was intact. The methodology used for inducing uveitis in animals has been described previously [27]. Follow-up duration ranged from 6 to 57 days. Based on the experimental protocols, animals were euthanized either after ophthalmoscopical resolution of symptoms of active uveitis (1-2 weeks after challenge) or after the temperature difference between the uveitic eye and the fellow eye decreased to a maximum of 0.2°С at consecutive time points of the follow. Animals were euthanized by intravenous injection of thiopental sodium (50 mg/kg). Thereafter, enucleated eyes were fixated in 10% formalin, embedded in paraffin, cut into 5-?m sections, stained with hematoxylin and eosin, and sent for examination of histomorphological features under the light microscope. Histomorphological studies were conducted at the Pathology and Electronic Microscopy Laboratory of the Filatov Institute. Images were captured at various magnifications using a PowerShot A480 camera (Canon Inc., Tokyo, Japan) attached to a Laboval 4 light microscope (Carl Zeiss, Jena, Germany). Results Histologically, the fellow eye enucleated on day 6 was of a normal structure typical for a healthy rabbit eye. This was true for all the anterior eye structures (the cornea, iris and ciliary body) and the posterior segment. No pathological change was observed in the choroid, vitreous or retina of the fellow eye at the end of day 6 after unilateral intravitreal challenge (Fig. 1).
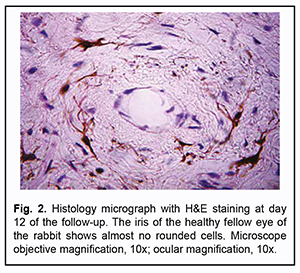
Over the second week after intravitreal challenge, the fellow eyes showed normal histology with no accumulation of inflammatory cells in the iris or ciliary body (Fig. 2).
Fig. 3 shows the cellular composition of the ciliary body on day 10. The ciliary body was composed of elongated cells, often dendritic cells with or without melanin pigment. These were mostly fibroblasts and melanocytes. However, inflammatory cells (lymphocytes, plasmocytes or monocytes) were either rare or absent not only on day 6 but also on day 10 and day 13. This indicated an absence of fellow eye response to the anterior and intermediate uveitis induced in the affected eye.
On the second week after intravitreal challenge, the retina and uvea again showed normal histology, with no pathological changes in the choroid (Fig. 4).
We noted no difference in histology of the fellow eye between the rabbits euthanized at the end of week 1 and at the end of week 2. Therefore, over two weeks after intravitreal challenge for induction of the anterior and intermediate uveitis, no pathological structural change was observed in the fellow eye. This rules out sympathetic ophthalmia as a possible cause of an increase in temperature in the fellow eye and stresses that such a response of the autonomic nervous system was only functional. In addition, we examined the morphology of the fellow eye in the rabbit after two weeks post intravitreal challenge for induction of the anterior and intermediate uveitis in order to rule out sympathetic ophthalmia as a possible cause of an increase in temperature in this period. At time points after two weeks post intravitreal challenge, the anatomical structures of the fellow eye (the cornea, iris and the ciliary body) maintained normal histology, with no signs of cyclitis or inflammation originating at other locations (Fig. 5). In addition, the ciliary body was composed of pigmented and pigmentless epithelial cells and elongated pigmented and pigmentless stromal cells, mostly fibroblasts and melanocytes (Fig. 6).
The histologic section of the posterior segment (Fig. 7) showed generally normal histology, from the vitreous and the two vessels embedded in the vitreous which bring into effect trophic processes in the underlying retinal medullary ray and are seen at right, to the sclera seen at left.
Therefore, at time points after two weeks post intravitreal challenge for induction of the anterior and intermediate uveitis, there was an absence of fellow eye response to the anterior and intermediate uveitis induced in the affected eye. Discussion There have been numerous reports on local specific changes developing in the fellow eye, with their severity of these changes depending on the changes in the affected eye. These changes may be functional, biochemical or immune in nature. Oculo-ocular phenomenon can be described as a complex combination of signs characteristic for the response of the fellow eye in the unilateral eye disease of various origin [28]. The general finding in sympathetic ophthalmia is uveal granulomatous inflammation primarily by lymphocytes, surrounding macrophages, and some multinucleated giant cells [29]. In our histomorphological study of healthy fellow eyes in unilaterally induced uveitis, we have failed to find any above signs of sympathetic ophthalmia, despite an increase in ocular temperature in the first days after disease onset. In addition, we have failed to find notes on an increase in temperature in the healthy fellow eyes in most available reports on the studies on ocular temperature in a unilateral pathological process. For instance, on day 1 after glaucoma surgery, thermography found a significant increase in temperature in the operated eye, but not in the non-operated eye, with an abnormal difference in temperature between the two eyes [30]. Chernookova [28] studied oculo-ocular reactions in unilateral eye trauma with the use of thermography. She, however, did not compare the affected eye with the unaffected eye with regard to temperature, but used an oculo-labial temperature gradient, since the use of symmetric areas in the fellow eyes might be incorrect due to potential oculo-ocular reactions. As opposed to our study, the study by Chernookova [28] has been focused on sympathetic ophthalmia and anticipated potential development of oculo-ocular reactions. In a recent paper, Chen and Caspi [31] stressed that accurate and easy-to-use methods are required for better use of experimental autoimmune uveitis models, and these methods should be noninvasive to exclude the effect on disease development and progression. They proposed to use noninvasive clinical assessments by fundus examination and photography, optical coherence tomography, and functional evaluation by electroretinography, which are then compared to histopathology. Using these methodologies, they demonstrated that clinical variants of disease can be accurately evaluated both clinically and functionally, facilitating longitudinal follow-up and providing information that cannot be obtained by fundoscopy and histology alone [31]. Our study on temperature of the ocular surface in the projection of the ciliary body conforms to the proposed requirements for assessing experimental uveitis. In addition, the methodology was found to be sensitive enough to help us with the detection of a functional oculo-ocular reaction of the healthy fellow eye to the development of uveitis in the challenged eye. Our histomorphological study confirmed the functional nature of an increase in temperature in the fellow eye in the absence of any histomorphological changes. Moreover, we believe that, in uveitis studies, the contact method we have suggested for measuring ocular surface temperature is more reasonable and accurate than thermography that is widely used in ophthalmology. It is this contact methodology that allowed for detecting such a functional oculo-ocular reaction that, to the best of our knowledge, has not been described previously. Because tearing is a common symptom of uveitis [7, 32-35], and tear evaporation rate affects ocular surface temperature assessed by thermography [36-39], the latter method should be considered quite disadvantageous and inaccurate, whereas contact methods, advantageous for assessing local temperatures in uveitis.
References 1.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013 Nov;131(11):1405-12. 2.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001 Jul;80(4):263-70. 3.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. 4.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996 Sep; 80(9): 844–8. 5.Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012 Aug 29;7:57. 6.Astakhov IuS, Kuztetsova TI. [Laser flare photometry in clinical practice]. Oftalmologicheskie vedomosti. 2016;9(2):36-44. Russian. 7.Guney E, Tugal-Tutkun I. Symptoms and Signs of Anterior Uveitis. US Ophthalmic Rev. 2013;6(1):33-7. 8.Herbort CP, Guex-Crosier Y, de Ancos E, et al. Use of laser flare photometry to assess and monitor inflammation in uveitis. Ophthalmology. 1997 Jan;104(1):64-71. 9.Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. Jan-Feb 2005;50(1):27-47. 10.Tugal-Tutkun I, Herbort CP. Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol. 2010;30:453–64. 11.Wakefield D, Herbort CP, Tugal-Tutkun I, Zierhut M. Controversies in ocular inflammation and immunology laser flare photometry. Ocul Immunol Inflamm. 2010 Oct;18(5):334-40. 12.Vinogradov VI, Veretenov IS, Slezko VN, Pugach GI, Landa VA, Bol'shakova GI. [Some aspects of application of thermography in the rehabilitation of patients with dysfunction of the musculoskeletal and nervous systems]. Funktsionalnaia diagnostika. 2005;3:72-8. Russian. 13.Zabolotnyi DI, Rozenfeld LG, Kolotilov NN, et al. [Novel potential of infrared thermography in otolaryngology]. Zhurnal vushnykh, nosovykh i gorlovykh khvorob. 2005;5:2-5. Russian. 14.Zamechnik TV, Larin SI. [The potential of thermography in diagnosing varicose veins of the lower extremities]. Flebologiia. 2009;3:10-14. Russian. 15.Lakusta VN, Moraru AT. [Thermography and cryotherapy in ortopedic neurology]. Kishinev; 2005. Russian. 16.Markel AL, Vainer BG. [Infrared thermography in the diagnosis of breast cancer: review of foreign literature]. Terapevticheskii Arkhiv. 2005;77:57-61. Russian. 17.Yakupov AF, Anisimov AIu, Galimzyanov AF, et al. [The potential of thermography in diagnosing and treating patients with portal hypertension-associated hepatic cirrhosis]. Kazanskii meditsinskii zhurnal. 2008;89(6):842-6. Russian. 18.Berry RJ, Kennedy AD, Scott SL, Kyle BL, Schaefer AL. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can J Anim Sci. 2003;83:687–93. 19.Chidambaram R, Shrinuvasan S, Srikumar R, et al. Digital infrared thermal imaging: as adjuvant screening modality in medical specialties. Journal of Innovative Research and Solutions (JIRAS). 2015; 1(1):13-9. 20.Hildebrandt C, Raschner C, Ammer K. An overview of recent application of medical infrared thermography in sports medicine in Austria. Sensors (Basel). 2010;10(5):4700-15. 21.Kaczmarek M, Nowakowski A, Siebert J, et al. Infrared thermography: applications in heart surgery. Proc SPIE. 1999;3730:184–8. 22.Lahiri BB, Bagavathiappan S, Jayakumar T, et al. Medical applications of infrared thermography: a review. Infrared Phys Technol. 2012 Jul; 55(4): 221–35. 23.Ng EY. A review of thermography as promising non-invasive detection modality for breast tumor. Int J Therm Sci. 2009;48:849–59. 24.Ring EF. The historical development of thermometry and thermal imaging in medicine. J Med Eng Technol. Jul-Aug 2006;30(4):192-8. 25.Soffin CB, Morse DR, Seltzer S, et al. Thermography and oral inflammatory conditions . Oral Surg Oral Med Oral Pathol. 1983 Sep;56(3):256-62. 26.Dorokhova O, Zborovska O, Meng Guanjun, et al. Temperature of the ocular surface in the projection of the ciliary body in rabbits. J Ophthalmol (Ukraine). 2020;2(493):65–9. 27.Dorokhova O, Zborovska O, Meng Guanjun. Temperature of the ocular surface in the projection of the ciliary body early in induced non-infectious uveitis in rabbits. J Ophthalmol (Ukraine). 2020;3(494):47-52. 28.Chernookova VA. [Clinical and functional patterns of oculo-ocular reflexes in unilateral mechanical ocular trauma]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Moscow: Moscow Helmholtz Research Institute for Eye Diseases; 2007. Russian. 29.Chu XK, Chan CC. Sympathetic ophthalmia: to the twenty-first century and beyond. J Ophthalmic Inflamm Infect. 2013 Jun 1;3(1):49. 30.Lopatinskaia NR. [Remote thermography and pupil response analysis the diagnosis of primary open-angle glaucoma]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Saratov: Saratov State Medical University; 2012. Russian. 31.Chen J, Caspi RR. Clinical and Functional Evaluation of Ocular Inflammatory Disease Using the Model of Experimental Autoimmune Uveitis. Methods Mol Biol. 2019;1899:211-227. 32.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005 Apr;89(4):444-8. 33.Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol. 1959 May;47(5 Pt 2):155-70. 34.Mahabadi N, Kim J, Edens MA. Iritis. 2019 Jun 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. https://www.ncbi.nlm.nih.gov/books/NBK430909/ 35.Smith JR, Rosenbaum JT. Management of uveitis: A rheumatologic perspective. Arthritis Rheum. 2002 Feb;46(2):309-18. 36.Acharya UR, Tan JH, Koh JE, et al. Automated diagnosis of dry eye using infrared thermography images. Infrared Phys Technol. 2015; 71:263–71. 37.Craig JP, Singh I, Tomlinson A. The role of tear physiology in ocular surface temperature. Eye. Eye (Lond). 2000 Aug;14 (Pt 4):635-41. 38.Morgan PB, Tullo AB, Efron N. Infrared thermography of the tear film in dry eye. Eye (Lond). 1995;9 ( Pt 5):615-8. 39.Tan J-H, Ng EYK, Acharya UR. Evaluation of tear evaporation from ocular surface by functional infrared thermography. Med Phys. 2010 Nov;37(11):6022-34. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|