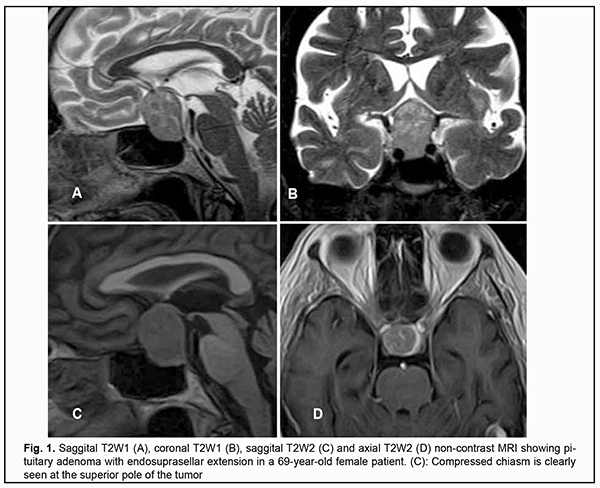
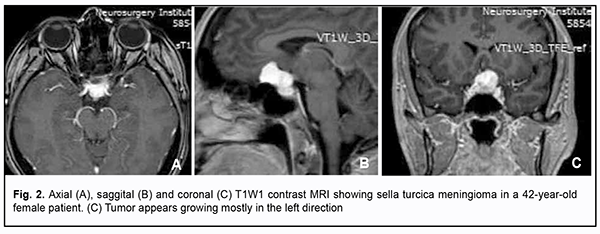
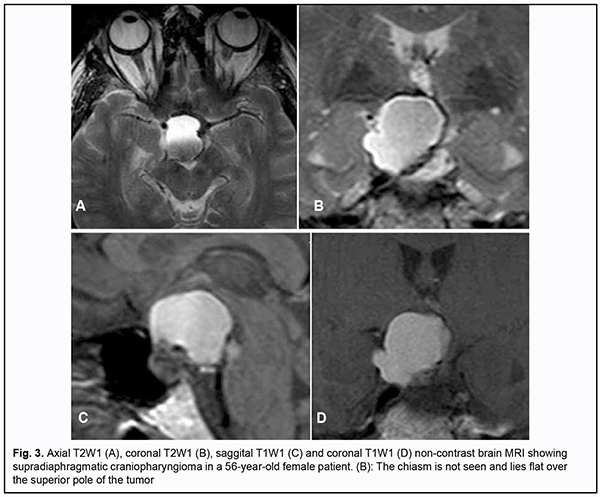
J.ophthalmol.(Ukraine).2020;4:23-27.
|
http://doi.org/10.31288/oftalmolzh202042327 Received: 07 May 2020; Published on-line: 27 August 2020
Clinical course of compressive optic neuropathy in skull-base tumors K.S. Iegorova, L.V. Zadoianyi, M.O. Guk, A.A. Chukov, O.V. Ukrainets, L.O. Danevych, A.O. Mumliev Romodanov Neurosurgery Institute, NAMS of Ukraine; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE: Iegorova KS, Zadoianyi LV, Guk MO, Chukov AA, Ukrainets OV, Danevych LO, Mumliev AO. Clinical course of compressive optic neuropathy in skull-base tumors. J.ophthalmol.(Ukraine).2020;4:23-27.http://doi.org/10.31288/oftalmolzh202042327 Background: Skull-base tumors (SBTs) of the middle and anterior fossae typically cause mass effect on the optic nerve/chiasm complex. The most common of these neoplasms are pituitary adenomas, meningiomas and craniopharyngiomas. Clinical manifestations of SBTs vary and depend on tumor involvement, nature of growth pattern, and rate of growth. Chiasmal compression is accompanied by a gradual decrease in visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA). Purpose: To investigate neuro-ophthalmological symptoms in patients with SBTs. Material and Methods: This retrospective study included the records of 500 patients who received treatment for SBT and loss of visual acuity and/or visual fields at the Romodanov Neurosurgery Institute during the period from 2017 through 2019. Patients underwent clinical and neurological, eye, and otoneurological examination (a routine otoneurological examination with assessment of cranial nerve function). Results: In patients with pituitary adenoma, chiasmal syndrome most commonly was symmetric (63.8%), and developed either gradually or (in a stroke-like course of tumor progression) abruptly. In addition, it was accompanied by a symmetric loss of visual acuity (77.1%), visual field defects (89.8%) and development of OA (50.5%). In patients with sella turcica meningioma, chiasmal syndrome most commonly was markedly asymmetric (65.7%), and accompanied by mild loss of visual acuity and visual fields in one eye, and severe or very severe visual acuity loss, residual visual field and OA in the fellow eye, as well as a particular compression of the anterior chiasm. Patients with supradiaphragmatic craniopharyngiomas had various amounts of loss of visual acuity and/or visual fields, and, most commonly, symmetric chiasmal syndrome and bilateral optic atrophy. In addition, the posterior chiasm (especially, the papillomacular bundle) was affected, which was manifested by bitemporal central scotoma (33.3%). Conclusion: We identified ophthalmological features of disease course in various histologic types of skull-base tumors. Loss of visual acuity and/or visual fields was an early and major symptom in the clinical picture of disease. Keywords: skull-base tumors, chiasmal syndrome, compressive optic atrophy
Introduction Skull-base tumors (SBTs) of the middle and anterior fossae typically cause mass effect on the optic nerve/chiasm complex. The most common of these neoplasms are pituitary adenomas, meningiomas and craniopharyngiomas [1, 2, 3, 4]. Chiasmal compression is accompanied by a gradual decrease in visual acuity, bitemporal visual field defects and development of primary descending optic atrophy (OA) [5, 6]. Clinical manifestations of SBTs vary and depend on tumor involvement, nature of growth pattern, and rate of growth. Impaired visual functions are observed in 67.8-83% of patients. Specifically, reduced visual acuity and visual field impairment have been found in 38-68.5% and 68-70%, respectively, of patients [5, 7, 8, 9]. 30-88.9% of cases manifest loss of visual acuity or visual fields [5, 9, 10]. Late-diagnosed but mostly benign processes causing compression of the optic nerve/chiasm complex may result in temporary or permanent visual function loss leading to visual disability. Prolonged chiasmal compression results in the development of OA in 26.7% to 72% of patients, leading to blindness in 3.5% to 25% of cases [4, 10, 11, 12]. Significant or rapidly progressive loss of visual acuity and/or visual fields in the presence of a small tumor may suggest malignancy. Despite progress in neuroimaging techniques, the number of new annually registered cases of primary optic atrophy (OA) associated with SBT and partial or complete loss of visual acuity and/or visual fields, is steadily increasing, making the issue increasingly important [5, 10, 12]. The purpose of the study was to investigate neuroophthalmological symptoms in patients with skull-base tumors. Material and Methods This retrospective study included the records of 500 patients (1000 eyes; 270 (54%) women and 230 (46%) men; aged 14 to 74 years; mean age, 51 ± 0.8 years) who received treatment for SBT and loss of visual acuity and/or visual fields at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2019. The inclusion criterion was surgical evidence of SBT. Patients underwent clinical and neurological, eye, and otoneurological examination (a routine otoneurological examination with assessment of cranial nerve function). Instrumental and laboratory studies were conducted. Neuroimaging included sella turcica X-ray study with AXIOM Iconos R100 (Siemens) or Radrex-I (Toshiba) in 72 patients, brain magnetic resonance imaging (MRI) with a 1.5-T MRI system (Intera 1.5T/I system, Philips Medical Systems, Best, the Netherlands) in all patients, and computed tomography (CT). The MRI of brain and pituitary gland were obtained using T1-weighted image (WI) and T2WI. Neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, static automated and kinetic perimetry, and direct and indirect ophthalmoscopy. Best-corrected visual acuity was classified as normal (1.0), mild impairment (0.7-0.9), moderate impairment (0.4-0.6), severe impairment (0.1-0.3), and very severe impairment (< 0.1). Static automated perimetry (SAP) was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) using the neurological 30-2 threshold test program and Neuro screening program. Aside from defect localization, the arithmetic mean of the sensitivity loss, the mean defect (MD), was used to assess visual field loss severity. Visual field loss severity was classified as “no visual field loss” (Grade 0; normal visual field), mild visual field loss (Grade 1; MD, –2 dB to –4 dB), moderate visual field loss (Grade 2; MD, –4 dB to –12 dB), severe visual field loss (Grade 3; MD, –12 dB to –20 dB), and very severe visual field loss (Grade 4; MD, worse than –20 dB). The visual field loss was classified as very severe if it was not possible to assess visual fields due to the extremely poor visual function. A chiasmal syndrome was considered symmetric if both eyes had the same grade of visual field loss. In addition, a chiasmal syndrome was considered asymmetric if the difference between eyes in grade of visual field loss severity was 1, and it was considered markedly asymmetric if the difference was 2 or greater. Eye movements in each of the four directions of gaze were assessed. Visual acuity was assessed with a diaphragm if there was mydriasis caused by oculomotor nerve palsy. This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. Results are presented as the mean and standard deviation (M ± SD). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ≤ 0.05 was assumed. Results and Discussion Loss of visual acuity and/or visual fields was found in 500 (100%) patients. Pituitary adenoma (PA) was the most common tumor-causing loss of visual acuity or visual fields (420 patients; 84%; Fig. 1), followed by sella turcica meningioma (35 patients; 7%; Fig. 2), craniopharyngioma (33 patients; 6%; Fig. 3), chiasmal glioma (8 patients; 2%), germinoma and teratoma (4 patients; 1%). There was surgical evidence both of suprasellar extension of the SBT and of compression of the optic nerve/chiasm complex in all patients.
All the 420 patients with PA exhibited loss of visual acuity in one or two eyes: in 96 (22.9%%) patients, only one eye had a VA lower than 1.0; in 268 (63.8%), both eyes had a VA lower than 1.0; in 32 (7.6%), one eye had a VA lower than 1.0, and another, a VA lower than 0.1; in 24 (5.7%), both eyes had a VA lower than 0.1. Best-corrected visual acuity was normal (1.0) in 96 (11.4%) eyes, mildly impaired (0.7-0.9) in 224 (26.6%) eyes, moderately impaired (0.4-0.6) in 226 (26.9%) eyes, severely impaired (0.1-0.3) in 214 (25.5%) eyes, and very severely impaired (< 0.1) in 80 (9.6%) eyes. In addition, 70 eyes (8.3%) were blind and 5 patients were bilaterally blind. Static perimetry found no changes in 43 (5.2%) eyes. Temporal hemianopia (either complete or partial) only was the commonest field defect (385 eyes; 45.9%), followed by temporal hemianopia with central scotoma (205 eyes; 24.6%), central scotoma only (58 eyes; 6.9%), and residual visual field in the nasal inner quadrant, with a loss of central vision (79 eyes; 9.5%). Visual field was not measurable due to extremely low visual function in 70 (8.3%) eyes. Loss of sensitivity to light was classified as mild in 132 (15.7%) eyes, moderate in 416 (49.5%) eyes, and severe and very severe in 174 (20.7%) eyes and 75 (8.9%) eyes, respectively. No loss of sensitivity to light was seen in 43 (5.1%) eyes. Ophthalmoscopy found primary descending OA in 212 (50.5%) patients. Of these, 188 patients (376 eyes) exhibited bilateral OA, and 24 patients (24 eyes), unilateral OA. Symmetric, asymmetric and markedly asymmetric chiasmal syndrome was found in 268 (63.8%), 114 (27.1%), and 38 (9.1%) patients, respectively. Ocular motility disorders (OMD) were found in 34 (8.1%) patients. Of these patients, 6 (17.6%) had isolated unilateral CNIII (oculomotor nerve) palsy, 19 (55.9%) had isolated unilateral CNVI (abducens nerve) palsy, and 9 (26.5%) had combined oculomotor and abducens nerve palsy. Sella turcica meningioma was found in 35 (100%) patients. Of these, 8 (22.9%) had the tumor with optic canal involvement. In addition, the commonest complaint was gradually decreased vision for 3 months to 2 years. Visual acuity loss was mostly asymmetric: in 15 (42.9%) patients, one eye had a VA of 1.0, and another, a VA lower than 1.0; in 9 (25.7%), both eyes had a VA lower than 1.0; in 8 (22.9%), one eye had a VA of 1.0, and another, a VA lower than 0.1; and in 3 (8.6%), one eye had a VA lower than 1.0, and another, a VA lower than 0.1. Best-corrected visual acuity was normal (1.0) in 23 (32.9%) eyes, mildly impaired (0.7-0.9) in 11 (15.7%) eyes, moderately impaired (0.4-0.6) in 9 (12.8%) eyes, severely impaired (0.1-0.3) in 17 (24.3%) eyes, and very severely impaired (< 0.1) in 10 (14.3%) eyes. Temporal hemianopia (either complete or partial) only was the commonest field defect (23 eyes; 32.9%), followed by temporal hemianopia with central scotoma (13 eyes; 18.6%), residual visual field in the inner half (10 eyes; 14.3%), residual visual field in the outer half (5 eyes; 7.1%), nasal hemianopia (4 eyes; 5.7%), lower hemianopia (2 eyes; 2.9%), and central scotoma only (1 eye; 1.4%). In addition, no visual field defect was found in 11 (15.7%) eyes. Visual field was not measurable due to extremely low visual function in 1 (1.4%) eye. Loss of sensitivity to light was classified as mild in 22 (31.4%) eyes, moderate in 9 (12.9%) eyes, and severe and very severe in 13 (18.6%) eyes and 15 (21.4%) eyes, respectively. No loss of sensitivity to light was seen in 11 (15.7%) eyes. Ophthalmoscopy found OA in 31 (88.6%) patients. Of these, 28 patients (28 eyes) exhibited unilateral OA, and 3 patients (6 eyes), bilateral OA. Symmetric, asymmetric and markedly asymmetric chiasmal syndrome was found in 3 (8.6%), 9 (25.7%), and 23 (65.7%) patients, respectively. Ocular motility disorders were found in two patients. These two had isolated unilateral partial CNIII palsy. Among the 33 patients with supradiaphragmatic craniopharyngioma, symmetric chiasmal syndrome was the most common (19 patients; 57.6%), followed by asymmetric (8 patients; 24.2%) and markedly asymmetric (6 patients; 18.2%) chiasmal syndrome. In 12 (36.4%) patients, only one eye had a VA lower than 1.0; in 11 (33.3%), both eyes had a VA lower than 1.0; in 3 (9.1%), one eye had a VA of 1.0, and another, a VA lower than 0.1; in 4 (12.1%), one eye had a VA lower than 1.0, and another, a VA lower than 0.1; and in 3 (9.1%), both eyes had a VA lower than 0.1. Best-corrected visual acuity was normal (1.0) in 15 (22.7%) eyes, mildly impaired (0.7-0.9) in 8 (12.1%) eyes, moderately impaired (0.4-0.6) in 13 (19.7%) eyes, severely impaired (0.1-0.3) in 18 (27.3%) eyes, and very severely impaired (< 0.1) in 12 (18.2%) eyes. Visual field defects were found in all eyes with supradiaphragmatic craniopharyngioma: central temporal scotoma was the commonest field defect (23 eyes; 32.9%), followed by temporal hemianopia (either complete or partial) only (12 eyes; 18.2%), temporal hemianopia with central scotoma (11 eyes; 16.6%), homonymous hemianopia (10 eyes; 15.2%), residual visual field in the inner half (5 eyes; 7.6%), and central scotoma only (2 eyes; 3%). In addition, visual field was not measurable in 4 (6.1%) eyes. Loss of sensitivity to light was classified as mild in 15 (22.7%) eyes, moderate in 17 (25.8%) eyes, and severe and very severe in 16 (24.2%) eyes and 18 (27.3%) eyes, respectively. Ophthalmoscopy found OA in 25 (75.8%) patients. Of these, 20 patients (40 eyes) exhibited bilateral OA, and 5 patients (5 eyes), unilateral OA. OMD were found in 3 patients. Of these patients, one had isolated unilateral partial CNIII palsy and two had isolated unilateral partial CNVI palsy. Therefore, the major sign of SBT is a gradual development of chiasmal syndrome, which is accompanied by decreased visual acuity, bitemporal visual field defects and development of primary compressive OA. Our findings for a large sample of SBTs of the middle and anterior fossae are in general agreement with those from the literature; however, most of these studies reported on significantly smaller samples [3, 4, 6, 7, 8, 10]. We found significant differences among pituitary adenoma, sella turcica meningioma and supradiaphragmatic craniopharyngioma groups (groups of the three most common skull-base tumors of the middle and anterior fossae) with regard to the features of the chiasmal syndrome and compression of the optic nerve/chiasm complex. Pituitary adenoma was most commonly associated with a symmetric chiasmal syndrome (63.8%), which developed either gradually or gradually or (in a stroke-like course of tumor progression) abruptly. In addition, it was accompanied by a symmetric loss of visual acuity (77.1%), visual field defects (89.8%) and development of OA (50.5%). In patients with sella turcica meningioma, chiasmal syndrome most commonly was markedly asymmetric (65.7%), developed for a long time (several years) and gradually, and accompanied by mild loss of both visual acuity and visual fields in one eye, and severe or very severe visual acuity loss, residual visual field and OA in the fellow eye. The principal pathogenetic mechanism for optic neuropathy relates to compression of the optic nerve/chiasm complex (mostly, of the anterior chiasm). Patients with supradiaphragmatic craniopharyngiomas had various amounts of loss of visual acuity and/or visual fields, and, most commonly, symmetric chiasmal syndrome and bilateral optic atrophy. In addition, the posterior chiasm (especially, the papillomacular bundle) was affected, which was manifested by bitemporal central scotoma (33.3%) and homonymous hemianopia (15.2%). The pathogenesis of impaired visual functions in patients with these tumors relates to chiasmal compression by a cystic tumor component, devascularization of the optic chiasm, and chiasmal tumor growth. Thus, there are ophthalmological features of disease course in various histologic types of skull-base tumors. Loss of visual acuity and/or visual fields was an early and major symptom in the clinical picture of disease. The role of the ophthalmologist in the early diagnosis of and monitoring of patients with skull-base tumors should be noted.
References 1.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015; 76(3):210-9. 2.Predoi D, Badiu C, Alexandrescu D, Agarbiceanu C, Stangu C, Ogrezeanu I, Ciubotaru V, Dumitrascu A, Constantinescu Al. Assessment of compressive optic neuropathy in long-standing pituitary macroadenomas. Acta Endocrinologica (Buc). 2008; 4(1):11-22. 3.Ekpene U, Ametefe M, Akoto H, Bankah P, Totimeh T, Wepeba G, Dakurah T. Ekpene U, et al. Ghana Med J. 2018; 52(6):79-83. 4.Masaya-anon P, Lorpattanakasem J. Intracranial tumors affecting visual system: 5-year review in Prasat Neurological Institute. J Med Assoc Thai. 2008; 91(4):515-19. 5.Foroozan R. Chiasmal syndromes. Curr Opin Ophthalmol. 2003; 14(6):325-31. 6.Astorga-Carballo A, Serna-Ojeda JC, Camargo-Suarez MF. Chiasmal syndrome: Clinical characteristics in patients attending an ophthalmological center. Saudi J Ophthalmol. 2017; 31(24):229-33. 7.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 8.Wadud SA, Ahmed S, Choudhury N, Chowdhury D. Evaluation of ophthalmic manifestations in patients with intracranial tumours. Mymensingh Med J. 2014 Apr;23(2):268-71. 9.Sefi-Yurdakul N. Sefi-Yurdakul N. Int J Ophthalmol. 2015 Aug 18;8(4):800-3. doi: 10.3980/j.issn.2222-3959.2015.04.28. 10.Tagoe NN, Essuman VA., Fordjuor G, Akpalu G, Bankah P, Ndanu T. Neuro-ophthalmic and clinical characteristics of brain tumours in a tertiary hospital in Ghana. Ghana Med J. 2015; 49(3):181-6. 11.Guk MO. [Diagnosis and comprehensive treatment of hormonally-inactive pituitary adenomas]. Dissertation for Doctor of Medical Sciences. Kyiv. 2017. 12.Serova NK. [Clinical neuroophthalmology. Neurosurgery aspects]. Tver’: Triada; 2011. Russian. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|