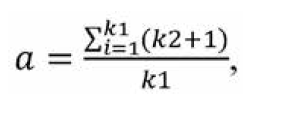J.ophthalmol.(Ukraine).2020;4:83-88.
|
http://doi.org/10.31288/oftalmolzh202048388 Received: 05 June 2020; Published on-line: 27 August 2020 Mathematical modelling in ranking timolol single active ingredient drugs by the efficacy criterion V.M. Sakovych1, O.V. Makarenko1, O.V. Kryvoviaz 2, Yu.O. Tomashevska2, V.M. Koval2 1Dnipropetrovsk Medical Academy, Ministry of Health of Ukraine; Dnipropetrovsk (Ukraine) 2 National Pirogov Memorial Medical University, Vinnytsya (Ukraine) E-mail: tomasevskau@gmail.com TO CITE THIS ARTICLE: Sakovych VM, Makarenko OV, Kryvoviaz OV, Tomashevska YuO, Koval VM. Mathematical modelling in ranking timolol single active ingredient drugs by the efficacy criterion. J.ophthalmol.(Ukraine).2020;4:83-88. http://doi.org/10.31288/oftalmolzh202048388 Background: Glaucoma is a disease that is associated with elevated intraocular pressure. Most commonly, single active agent beta-adrenoceptor blockers (particularly, timolol-only preparations) are used as a first-line therapy for primary open-angle glaucoma (POAG). Purpose: To compare the efficacy of various timolol-only preparations available in the Ukrainian pharmaceutical market as a monotherapy for POAG on the basis of expert assessment. Methods: Forty-four ophthalmologists from various Ukrainian regions participated in the questionnaire survey. The participants varied in length of service, academic degree and experience in managing the disease. Survey data were processed using the method of the prior ranking of factors. Results: Mathematic modeling was used to construct the bar chart of ranks from the processed results of the survey. The overall impact of any factor on the examined variable was assessed through the sum of ranks. Conclusion: The method of the prior ranking of factors was used to establish that, in the opinion of the surveyed ophthalmologists, CUSIMOLOL, ARUTIMOL and OFTAN TIMOLOL are the three most efficient timolol-only preparations for the treatment of POAG. Keywords: a priori ranking, glaucoma, Timolol drugs
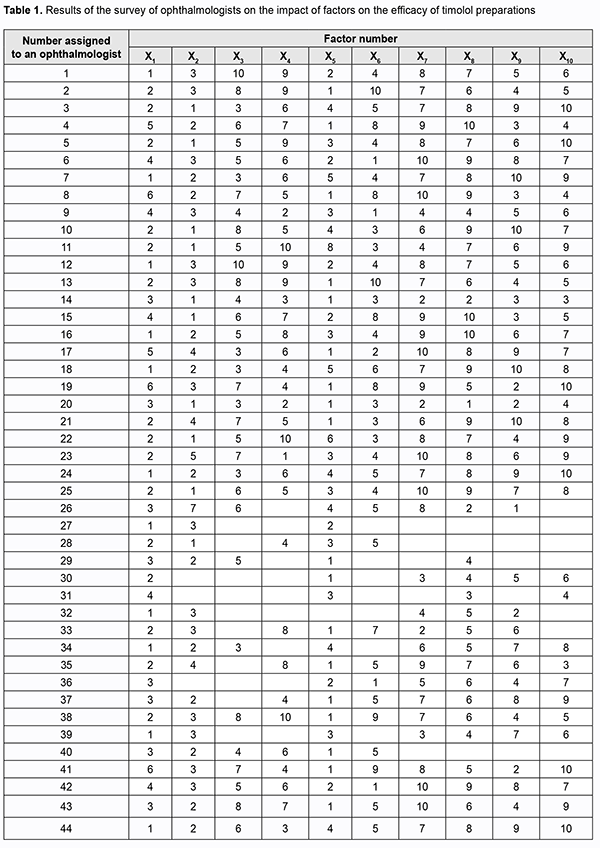
Introduction Glaucoma is a disease that is associated with elevated intraocular pressure (IOP) and results in optic nerve damage with progression to optic nerve atrophy which can lead to loss of visual functions and even blindness. Early disease is characterized by an absence of patient’s complaints, but as the disease progresses to more severe stages, the visual fields gradually constrict, and optic nerve atrophy becomes irreversible. In addition, the patient partially loses visual functions, which may limit his/her physical activity and affect his/her professional performance. Primary open-angle glaucoma (POAG) is the most common clinical form of the disease; it accounts for 70% to 92% of cases [1, 2]. The age at diagnosis of POAG has decreased with time [1]. This is an important social issue since an increase in disability rate for individuals of working age will result in negative economic consequences for the nation. Adequate choice of anti-glaucoma drugs and planning for use of funds for these agents are essential. Beta-adrenoceptor blockers and synthetic prostaglandin analogues are used as a first-line therapy to improve aqueous humor outflow, and are followed by adjunctive alpha adrenomimetics and cholinergic drugs if necessary. Most commonly, single active agent preparations, particularly, timolol-only preparations, are used as a first-line therapy for POAG. The purpose of this study was to compare the efficacy of various timolol preparations available in the Ukrainian pharmaceutical market as a monotherapy for POAG on the basis of expert assessment. Material and Methods We examined the national medication register and identified timolol-only preparations used in POAG therapy. In addition, we conducted a questionnaire survey to identify a relative efficacy of timolol-only preparations in the treatment of POAG on the basis of expert assessment. Survey data were processed using the method of the prior ranking of factors [3,4]. Results and Discussion Forty-four ophthalmologists from various Ukrainian regions participated in the survey. The participants varied in length of service, academic degree and experience in managing the disease. They were proposed to determine the trademarks under which they most commonly clinically administer timolol as a monotherapy and which are most effective for POAG, on the basis of their personal theoretical knowledge and practical experience. The main efficacy criteria were a decrease in IOP, minimal daily IOP fluctuations, duration of hypotensive action, safety and easy administration. It is these characteristics that were taken into account by the ophthalmologists involved in assessment. We examined the national medication register and identified 10 timolol-only preparations registered in Ukraine as of January 20, 2019 [5]. For convenience of data processing, the identified preparations were assigned corresponding designations: ARUTIMOL® 5 mg/ml eye drop solution supplied in a 5-ml dropper bottle enclosed in a carton (Dr. GERHARD MANN Chem.-Pharm. Fabrik, GmbH, Germany, and Laboratoire CHAUVIN, France), factor X1; CUSIMOLOL® 0.5% eye drop solution supplied in a 5-ml dropper bottle enclosed in a carton (Alcon Cusi SA, Spain), factor X2; NORMATIN 0.5% eye drop solution supplied in a 5-ml dropper bottle enclosed in a carton (EIPI Co., Egypt), factor X3; OCUMED 0.5% eye drop solution supplied in a 5-ml or 10-ml plastic dropper bottle enclosed in a pack (PROMED EXPORTS Pvt Ltd, India), factor X4; OFTAN® TIMOLOL 0.5% eye drops supplied in a 5-ml dropper bottle enclosed in a carton (Santen AT, Finland), factor X5; OFTIMOL® 0.5% 5 mg/ml eye drop solution supplied in a 5-ml or 10-ml plastic bottle enclosed in a carton (FARMAK JSC, Ukraine), factor X6; TIMOLOL 0.5% eye drop solution supplied in a 5-ml bottle with a dropper cap enclosed in a pack (Elegant India, India), factor X7; TIMOLOL-DARNYTSIA 5 mg/ml eye drop solution supplied in a 5-ml or 10-ml bottle enclosed in a pack (Darnytsia Pharmaceutical Company JSC, Ukraine), factor X8; TIMOLOL MALEAT 5 mg/ml eye drop solution supplied in a 5-ml or 10-ml bottle enclosed in a pack (FARMAK JSC, Ukraine), factor X9; and TIMOLOL MALEAT 5 mg/ml eye drop solution supplied in a 5-ml or 10-ml glass bottle with a dropper cap enclosed in a pack (BIOFARMA, Kyiv, Ukraine), factor X10. The participants were asked to arrange the preparations in descending order of efficacy on the basis of personal clinical experience, and to assign them ranks from 1 (best characteristics) to 10 (worst characteristics). Results of the survey are summarized in Table 1.
Since some participants failed to distinguish the effect of factors on the studied characteristic and assigned equal ranks to some factors, we had to transform the ranks. For this purpose, we used the following formula:
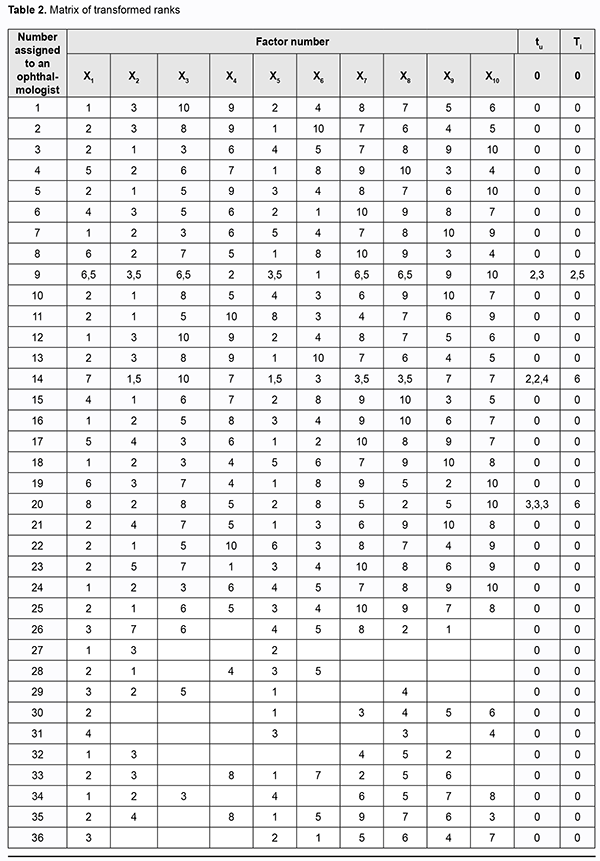
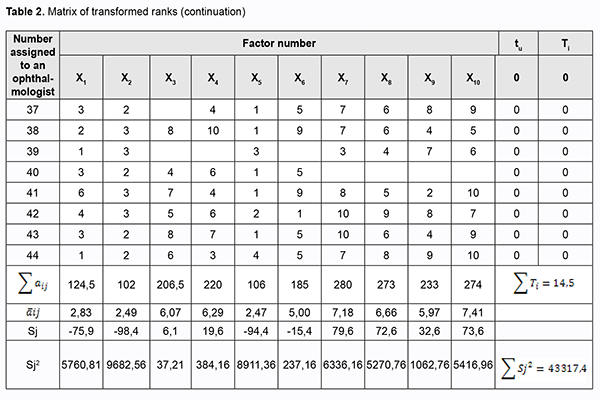
where a is the transformed rank; k1 is a number of equal ranks in the corresponding rank group for the participant; and k2 is a number of factors preceding a group of factors with equal ranks. We created a matrix of transformed ranks on the basis of (a) results of expert assessment of timolol preparation efficacy, (b) primary processing of these results and (c) rank transformation (Table 2).
The survey results were processed in the following way. First, the sum of ranks for each factor was determined:
where m is the number of survey participants. Second, the deviation from the mean rank sum was calculated:
where Sj is the deviation of rank sum of factor j from the mean rank sum; k is the number of factors,
and1/k is the mean rank sum. Kendall’s coefficient of concordance was used to assess the level of agreement between experts:
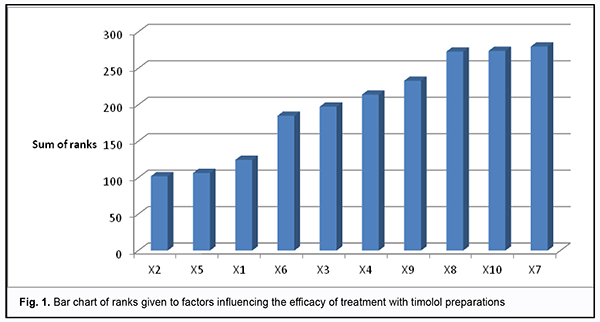
is the sum of squares for the deviation of rank sum of factor j from the mean rank sum; k is the number of factors, and m k is the number of experts. In our case, the coefficient of concordance was 0.3. A low value of the coefficient likely can be explained by that experts experienced difficulties in ranking due to a large number of factors. The chi-square test was used to assess the statistical significance of the coefficient of concordance. For a sample size of 10, or 10-1=9 degrees of freedom, we obtained a tabulated chi-square statistic of 16.9 at а=0.05. Because the calculated chi-square statistic (?2calc=107.57) was greater than the tabulated chi-square value at the 0.05 level of significance, expert opinions agreed with each other. With regard to expert assessment of timolol-only preparations used in POAG therapy, mathematic modeling was used to construct the bar chart of ranks from the processed results of the survey (Fig. 1).
The overall impact of any factor on the examined variable was assessed through the sum of ranks: the less the sum of ranks, the greater the impact of the examined factor. Therefore, in the opinion of experts, the following preparations were the best with regard to efficacy of treatment: CUSIMOLOL® 0.5% eye drop solution supplied in a 5-ml dropper bottle enclosed in a carton (Alcon Cusi SA, Spain); ARUTIMOL® 5 mg/ml eye drop solution supplied in a 5-ml dropper bottle enclosed in a carton (Dr. GERHARD MANN Chem.-Pharm. Fabrik, GmbH, Germany, and Laboratoire CHAUVIN, France); OFTAN® TIMOLOL 0.5% eye drops supplied in a 5-ml dropper bottle enclosed in a carton (Santen AT, Finland). Conclusion First, we examined the national medication register and identified 10 timolol-only preparations registered under various trade names for the treatment of glaucoma in Ukraine. Second, the method of the prior ranking of factors was used to establish that, in the opinion of the surveyed ophthalmologists, CUSIMOLOL, ARUTIMOL and OFTAN TIMOLOL are the three most efficient timolol-only preparations for the treatment of POAG. References 1.Kryvov’iaz OV. [Glaucoma patients’ quality of life: social and pharmacoeconomic aspects]. Dnipro: Litograf; 2017. Ukrainian. 2.European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Savona: PubliComm; 2014. 3.Groshovyi TA, Martseniuk VP, Kucherenko LІ, et al. [Mathematic planning of experiments in pharmaceutical research]. Ternopіl: Ukrmedkniga; 2008. Ukrainian. 4.Babintseva LIu. [Expert survey to assess the efficacy of medications]. Medychna informatika ta inzheneriia. 2014;1:21-3. Ukrainian. 5.The State Register of Medicinal Products of Ukraine. Available from: http://www.drlz.com.ua. Ukrainian. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|