J.ophthalmol.(Ukraine).2020;3:47-52.
|
http://doi.org/10.31288/oftalmolzh202034752 Received: 13 March 2020; Published on-line: 24 June 2020 Changes in temperature of the ocular surface in the projection of the ciliary body in the early stages of induced non-infectious uveitis in rabbits Dorokhova O., Zborovska O., Meng Guanjun SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Odesa (Ukraine) E-mail: dorochovaa@gmail.com TO CITE THIS ARTICLE: Dorokhova O., Zborovska O., Meng Guanjun. Changes in temperature of the ocular surface in the projection of the ciliary body in the early stages of induced non-infectious uveitis in rabbits. J.ophthalmol.(Ukraine).2020;3:47-52. http://doi.org/10.31288/oftalmolzh202034752 Background: It is an important challenge to develop objective methods for assessing intraocular inflammation. Purpose: To determine temperatures of the ocular surface in the projection of the ciliary body in rabbits with induced non-infectious anterior and intermediate uveitis in the first five days of the disease. Material and Methods: Temperatures of the ocular surface in the projection of the ciliary body were determined in seventeen Chinchilla rabbits with induced non-infectious anterior and intermediate uveitis. Results: At day 1, there was significant difference in temperature of the ocular surface in the projection of the ciliary body between challenged eyes and eyes of intact rabbits (р=0.002) and between fellow eyes and eyes of intact rabbits (р=0.005), but not (р=0.12) between the challenged eye and the fellow eye (35.7°С (1.1) vs 35.0°С (0.9)). At day 3, temperature for the challenged eye was 36.0°С (0.7), and for the fellow eye, 34.9°С (0.5), and the difference was not significant (р = 0.09), but there was significant difference between challenged eyes and eyes of intact rabbits. At day 5, a significant difference in temperature of the ocular surface appeared between the challenged eye and the fellow eye (36.0°С (0.7) vs 34.7°С (0.5); р=0.04), and there was still a significant difference between challenged eyes and eyes of intact rabbits (р=0.008). There was no significant difference in body temperature between challenged animals at day 1 and intact animals (39.1°С vs 39.1°С; р=0.75) and between challenged animals at day 3 and intact animals (39.2°С vs 39.1°С; р=0.58). Conclusion: On day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, the temperature in ciliary body projection onto the ocular surface increased to 35.7°С (р = 0.002) for the challenged eyes and to 35.0°С (р=0.05) for the fellow eyes compared to eyes of intact rabbits, which is likely to be caused by the response of the autonomic nervous system in the presence of initiation of inflammatory process. On day 5, a significant difference appeared in the ocular surface temperature between the challenged eyes and fellow eyes (36.0°С vs 34.7°С; р = 0.04), which is likely to be caused by decreased temperature response of the autonomic nervous system as well as by a gradual decrease in temperature of the fellow eye. Keywords: uveitis, objectively assess inflammation, ocular surface temperature
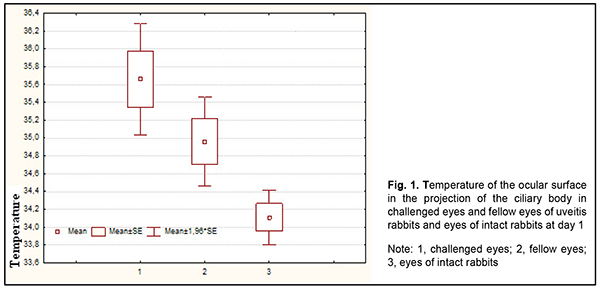
Introduction The incidence of uveitis varies from 17 to 24 per 100,000, and the prevalence is between 38 to 204 per 100,000 population, which means the disease can be classified as a rare (orphan) disease. Uveitis has been demonstrated to cause between 5% and 20% of cases of legal blindness in the United States and the European Union; in addition, it is the fourth most common cause of visual disability in developing countries [1-5]. Selecting the time for withdrawal of anti-inflammatory therapy in anterior uveitis is a challenge. The time of clinical recovery may not coincide with the time of actual recovery due to sluggish inflammation which has not been duly treated most commonly due to the absence of diagnostic criteria for this subclinical inflammation [6]. The degree of uveitis activity is the primary indication for therapy, and a change in the degree of this activity is an important basis for assessment whether the therapy is efficient or not. Nowadays laser flare photometry is the only available objective method of determining the activity and degree of inflammation. The laser flare meter has been introduced for quantification of anterior chamber protein and cells, which allows detecting even subclinical inflammation. The technique is employed both for disease diagnosis and monitoring [7-12]. It is, however, unreliable in eyes with poor mydriasis or advanced cataract, and another limitation is the absence of consensus on the clinical efficacy of the technique [13, 14]. Studies on local body temperatures improve the potential for analysis of biological processes in organs and tissues of the body. Heat energy is constantly produced in the body and the amount of heat energy produced depends on the intensity of metabolic and inflammatory processes as well as state of circulation [15]. It is an important challenge to develop easy, cost-effective and reliable objective methods for assessing intraocular inflammation: not only patient-specific treatment strategy, but also assessment of clinical trial results and development of clinical guidelines and protocols depend on it. Local measurements of temperature responses have been successfully used in other fields of medicine, and seem promising for objective assessment of ocular inflammation. In our most recent study, we have found that the pattern of heat exchange in intact rabbit’s ocular surface (temperature of the ocular surface in the projection of the ciliary body is influenced by autonomic thermoregulation and is relatively stable at small environmental temperature variations) allows for modeling of unilateral ocular pathological processes that would have a change in local body temperature in the projection of the ciliary body as an objective marker [16]. Therefore, the purpose of the study was to determine temperatures of the ocular surface in the projection of the ciliary body in rabbits with induced non-infectious anterior and intermediate uveitis in the first five days of the disease. Material and Methods Seventeen Chinchilla rabbits (34 eyes; weight, 2.5-3.0 kg) were included in this study. They were divided in two experimental series, series 1 (7 rabbits) and series 2 (10 rabbits). Animals were used in the experiments after being quarantined for 2 weeks. They were housed at the normal vivarium temperature of 18 до 25°С, and fed and watered conventionally. Non-infectious anterior or intermediate uveitis was induced in the right eye of each rabbit, while the left was intact. The Penkov and colleagues’ model [17] of experimental secondary (recurrent) toxic-and-allergic uveitis was modified and used in the study. Non-infectious uveitis was induced as follows: The animals were sensitized by injecting 1.0 mL sterile normal horse serum into the marginal ear vein at intervals of 24 hours for 5 days. Ten days thereafter, they were challenged by injecting 1.0 mL sterile normal horse serum intravitreally OD. The uveal inflammatory process in the form of anterior and intermediate uveitis developed a day thereafter. Our model differs from that of Penkov et al in that we introduce horse serum intravitreally rather than in the anterior chamber. Biomicroscopy, ophthalmoscopy, measurements of temperature of the ocular surface in the projection of the ciliary body and inflammation score were used to monitor the course of inflammation. Temperature of the ocular surface in the projection of the ciliary body was measured as described previously. In addition, the data obtained were compared with those from our previous study. The mean temperature of the ocular surface in the projection of the ciliary body in intact rabbits was 34.1°С (SD=1.4) [16]. A thermoelectric device [18] developed within the framework of the partnership agreement between the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring ocular surface temperature. Measurements were conducted every other day or every third day for rabbits of series 1 and every fourth to sixth day for rabbits of series 2. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Data are presented as mean ± standard deviation (SD). Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ? 0.05 was assumed. ANOVA and Newman-Keuls post-hoc analysis were used for comparisons of groups of different sizes. Results and Discussion Anterior and intermediate uveitis developed in the right eye a day after the intravitreal challenge with sterile normal horse serum. Details of the clinical findings and the relationship between clinical findings and ocular surface temperature will be reported in our future publications. There was no significant difference (р > 0.05) in room temperature between measurements made for animals with experimental uveitis (20.5°С (1.4)) and measurements made for intact animals (20.1°С (2.02)). There were mild but significant correlations of temperature of the ocular surface in the projection of the ciliary body with room temperature (r=0.27; p=0.0001) and body temperature (r=0.15; p=0.04). In addition, there was a mild correlation between body temperature and room temperature (r=0.37; p=0.0001). It is likely that mild but significant correlations found in this part of the experimental study could be explained by a greater number of ocular surface temperature measurements (N=189) compared to the study for eyes of intact rabbits (N=84). Throughout the follow-up period, there was no significant difference (р=0.104) in temperature of the ocular surface in the projection of the ciliary body between eyes of intact animals and healthy fellow eyes of challenged rabbits, although the temperature for the latter eyes was somewhat higher than for the former eyes (34.4°С (1.1) vs 34.1°С (1.4)). A day after the intravitreal challenge with sterile normal horse serum, a clinical picture of anterior and intermediate uveitis developed, and there was no significant difference (р=0.12) in temperature of the ocular surface in the projection of the ciliary body between the challenged eye and the fellow eye (35.7°С (1.1) vs 35.0°С (0.9)), although there was significant difference in temperature between challenged eyes and eyes of intact rabbits (р=0,002) and between fellow eyes and eyes of intact rabbits (р=0.005) (Fig. 1). This indicated an increase in temperature both for the challenged eye and for the fellow eye.
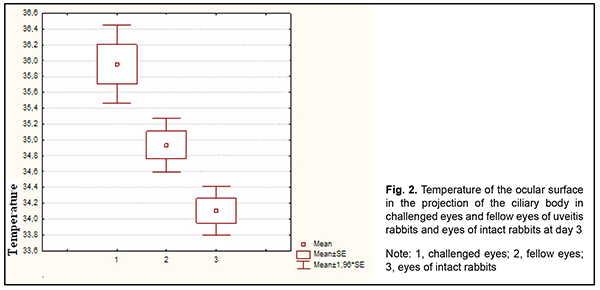
At day 3, temperature of the ocular surface in the projection of the ciliary body for the challenged eye was 36.0°С (0.7), and for the fellow eye, 34.9°С (0.5), but the difference was not significant (р = 0.09). In addition, the difference in temperature of the ocular surface in the projection of the ciliary body between challenged eyes and eyes of intact rabbits was significant (р=0.002), whereas that between fellow eyes and eyes of intact rabbits was not (р=0.17), which may indicate a tendency toward a decrease in temperature for the fellow eye (Fig. 2).
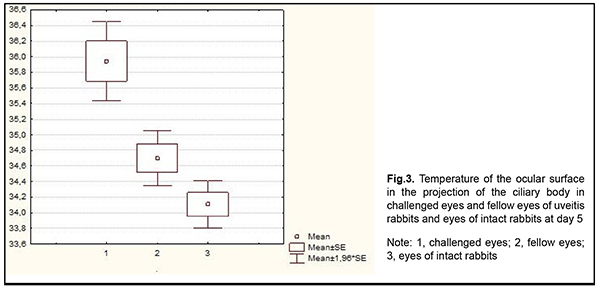
At day 5, a significant difference in temperature of the ocular surface appeared between the challenged eye and the fellow eye (36.0°С (0.7) vs 34.7°С (0.5); р=0.04), and there was still a significant difference between challenged eyes and eyes of intact rabbits (р=0.008). In addition, the difference in temperature of the ocular surface between fellow eyes and eyes of intact rabbits increased and was not statistically significant (р=0.3), indicating a continued tendency toward a decrease in temperature for the fellow eye (Fig. 3).
Although we expected systemic response in the form of increased body temperature in the first days of induced disease, there was no significant difference in body temperature between challenged animals at day 1 and intact animals (39.1°С vs 39.1°С; р=0.75) and between challenged animals at day 3 and intact animals (39.2°С vs 39.1°С; р=0.58). This absence of significant difference may indicate minimal systemic reaction to the development of uveitis. Aksionovа and colleagues [19] reported changes in immune status in rabbits (increased counts of white blood cells and neutrophils, increased phagocytic activity of neutrophils, and extensive phagocytosis in the peripheral blood) in the presence of clinical horse serum-induced autoimmune uveitis. We found no significant difference (р = 0.08) in body temperature between control animals (39.1°С) and challenged animals (39.0°С) within the follow-up period. Therefore, we observed an increase in temperature both in challenged eyes and in fellow eyes in the first days of induced non-infectious uveitis. Interestingly that on day 1 of experimental disease, no increase in body temperature was observed, but, along with an increased temperature of the challenged eye, there was an increase in the temperature of the fellow eye, with a significant difference from the temperature of eyes of intact animals. Since there was no increase in body temperature on day 1, an increase in the temperature of the fellow eye could not be caused by an increase in body temperature. Therefore, an increase in the temperature of the fellow eye at day 1 of experimental uveitis is most likely to be caused by the presence of so-called autonomic response of the fellow eye to temperature change. This response began decreasing from day 3, and on day 5 decreased to the level enabling a significant difference in temperature of the ocular surface between the challenged eye and the fellow eye. The presence of autonomic response of the fellow eye to temperature change should be taken in account when assessing the temperature of the ocular surface in the acute uveitis phase. A change in autonomic nervous system (ANS) activity is known to be one of the first stress-response phases. Vessels of the ciliary body, iris and choroid have both sympathetic and parasympathetic innervation [20]. Effects of the sympathetic system on changes in temperature have been demonstrated in a study [21] in which intradermal histamine injection was accompanied by an increase in limb temperature. Stewart and colleagues [22] demonstrated the use of eye temperature measured by infrared thermography as an indicator of ANS activity in bull calves. In addition, pain and anxiety exert effects on the sympathetic system and hot flash sensation [21]. It is likely that it was the combination of inflammation, impaired regulation of circulation, and pain sensation induced during the acute phase of uveitis that resulted in imbalance between the sympathetic and parasympathetic nervous systems and increased temperature of the fellow eye. Most rabbit studies on ocular surface temperature in health and or in any disease have been focused on measurements of intraocular temperature or corneal temperature [23-28]. The same tendency may be seen in human studies on temperature responses in uveitis; these studies have been focused on measurements of corneal and limbal temperatures [29-32] and even eyelid skin temperatures [33]. The only study assessing not only corneal temperature but also temperature of ciliary body projection on the ocular surface was that by Bakbardina [6]. We believe that, in uveitis studies (particularly, in anterior and intermediate uveitis studies), it is ciliary body projection on the ocular surface that is the most appropriate site for temperature measurements, since corneal temperature will not represent the true picture of disease. In order to undergo changes on the ocular surface, heat energy has to “pass a long distance” from the locus of inflammation in the iris, and, especially, in the ciliary body. However, temperature measurements in ciliary body projection onto the ocular surface will better reflect heat processes in the uvea due to reduced “heat losses”. Because the cornea is avascular, it is heated only passively, by heat expansion from the internal ocular structures (primarily from the uvea) through the aqueous in the anterior chamber. The ciliary body is a well-vascularized (as opposed to the cornea) part of the uvea which generates heat and is in direct contact with the sclera [34]. In addition, the thermal conductivity of the sclera is known to be higher than that of the cornea [35]. Therefore, temperature measurements in ciliary body projection onto the ocular surface will better reflect heat processes in the ciliary body (especially in the presence of ciliary body inflammation) compared to temperature measurements on the corneal surface. We believe a study by Rushton and colleagues [36] on the use of infrared thermography and infrared thermometry in the diagnosis of uveitis in horses is interesting with regard to ocular surface measurements in uveitis. Ocular temperatures of both eyes of horses with acute unilateral uveitis and normal horses were measured using thermometry and thermography. Measurements were conducted within three days from the detection of inflammation symptoms. They found that mean ocular temperature in uveitic eyes was higher than in contralateral eyes, which is in agreement with our findings. In addition, no statistically signi?cant in?uence of rectal temperature on ocular temperature was determined in either measurement method. Moreover, a statistically signi?cant in?uence of ambient temperature on the results of ocular thermometry was determined [28]. In that study and in our study measurements were conducted during the acute phase of uveitis. In our opinion, the results of that study could be influenced not only by technical characteristics of the used devices, but also some other factors. First, eye images were taken at 1 m distance, and it is difficult to prevent environmental effects (i.e., the influence of air current) at this distance. Second, target infrared thermometry is difficult to perform. Third, in some cases, it was not clear for how long the clinical signs of inflammation were present at the beginning of the study. Finally, in early acute uveitis, there could be the presence of autonomic response to temperature change. Conclusion First, on day 1 of induced non-infectious anterior and intermediate uveitis in rabbits, the temperature in ciliary body projection onto the ocular surface increased significantly to 35.7°С (р = 0.002) for the challenged eyes and to 35.0°С (р=0.05) for the fellow eyes compared to eyes of intact rabbits, which is likely to be caused by the response of the autonomic nervous system in the presence of initiation of inflammatory process. Second, on day 5 of induced non-infectious anterior and intermediate uveitis in rabbits, a significant difference appeared in the ocular surface temperature between the challenged eyes and fellow eyes (36.0°С vs 34.7°С; р = 0.04), which is likely to be caused by decreased temperature response of the autonomic nervous system as well as by a gradual decrease in temperature of the fellow eyes. References 1.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013 Nov;131(11):1405-12. 2.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001 Jul;80(4):263-70. 3.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990. 14:303–8. 4.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996 Sep; 80(9): 844–8. 5.Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012 Aug 29;7:57. 6.Bakbardina LM. [Thermometric diagnostics of inflammatory process in the anterior uvea]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Odesa: Filatov Institute of Eye Disease and Tissue Therapy; 1988. Russian. 7.Astakhov IuS, Kuztetsova TI. [Laser flare photometry in clinical practice]. Oftalmologicheskie vedomosti. 2016;9(2):36-44. Russian. 8.Guney E, Tugal-Tutkun I. Symptoms and Signs of Anterior Uveitis. US Ophthalmic Rev. 2013;6(1):33-7. 9.Herbort CP, Guex-Crosier Y, de Ancos E, et al. Use of laser flare photometry to assess and monitor inflammation in uveitis. Ophthalmology. 1997 Jan;104(1):64-71. 10.Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. 2005 Jan-Feb;50(1):27-47. 11.Tugal-Tutkun I, Herbort CP. Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol. 2010;30:453–64. 12.Wakefield D, Herbort CP, Tugal-Tutkun I, Zierhut M. Controversies in ocular inflammation and immunology laser flare photometry. Ocul Immunol Inflamm. 2010 Oct;18(5):334-40. 13.Gonzales CA, Ladas JG, Davis JL, Feuer WJ, Holland GN. Relationships Between Laser Flare Photometry Values and Complications of Uveitis. Arch Ophthalmol. 2001 Dec;119(12):1763-9. 14.Yeo TH, Ilangovan S, Keane PA, et al. Discrepancies in assessing anterior chamber activity among uveitis specialists. Jpn J Ophthalmol. 2016 May;60(3):206-11. 15.Chernookova VA. [Clinical and functional patterns of oculo-ocular reflexes in unilateral mechanical ocular trauma]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Moscow: Moscow Helmholtz Research Institute for Eye Diseases; 2007. Russian. 16.Dorokhova OE, Zborovska OV, Meng Guanjun. Temperature of the ocular surface in the projection of the ciliary body in rabbits. J. Ophthalmol. (Ukraine). 2020; 2(493):65–9. 17.Penkov MA, Saitov MA, Panchenko NV. [Certificate of authorship No. 1,601,629 (USSR). Method for inducing experimental toxic-and-allergic uveitis]. Issued 23.10.90, Bull. No. 39. 18.Anatychuck LI, Pasyechnikova NV, Zadorozhnyy OS, et al. Original device and approaches to the study of temperature distribution in various eye segments (experimental study). J. Ophthalmol. (Ukraine). 2015;6:50-3. 19.Aksionovа SV, Pyatayev NA, Malkina MV, et al. [Comparative examination of two methods for modeling autoimmune uveitis]. Mordovia University Bulletin. 2017; 27(3):428-39. Russian. 20.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015 Jan;5(1):439-73. 21.Sullivan DA, Stern ME, Tsubota K, Dartt DA, Sullivan RM, Bromberg BB (Eds). Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3: Basic Science and Clinical Relevance Part B. Adv Exp Med Biol. Vol. 506. Springer; US, 2002. 22.Stewart M, Webster JR, Stafford KJ, et al. Technical note: Effects of an epinephrine infusion on eye temperature and heart rate variability in bull calves. J Dairy Sci. 2010 Nov;93(11):5252-7. 23.Coles WH, Pandya RK, Anbar M, et al. Ocular surface temperature (ocular thermography) as a predictor of corneal wound healing. Invest Ophthalmol Vis Sci. 1988;29:313. 24.Fatt I, Forester JF. Errors in eye tissue temperature measurements when using a metallic probe. Exp Eye Res. 1972 Nov;14(3):270-6. doi: 10.1016/0014-4835(72)90013-9. 25.Freeman RD, Fatt I. Environmental influences on ocular temperature. Invest Ophthalmol. 1973 Aug;12(8):596-602. 26.Lorget F, Parenteau A, Carrier M, et al. Characterization of the pH and temperature in the rabbit, pig, and monkey eye: key parameters for the development of long-acting delivery ocular strategies. Mol Pharm. 2016 Sep 6;13(9):2891-6. 27.Rosenbluth RF, Fatt I. Temperature measurements in the eye. Exp Eye Res. 1977 Oct;25(4):325-41. 28.Schwartz B. Environmental temperature and the ocular temperature gradient. Arch Ophthalmol. 1965 Aug;74:237-43. 29.Antonchik SL. [Temperature characteristics of the eye in health and some pathological processes]. [Abstract of a Thesis for the Degree of Cand Sc (Biol)]. Tyumen: State Medical Academy; 2005. Russian. 30.Katargina LA. [Endogenous uveitis in infants: Clinical, functional and immunological features and management of complications]. [Abstract of a Dissertation for the Degree of Dr Sc (Med)]. Moscow: Helmholtz Research Institute of Eye Diseases; 1992. 31.Khvatova AV, Katargina LA, Lokhmanov VP, Zibarov IN. [Use of distant thermography in uveitis in children]. Vestn Oftalmol. Sep-Oct 1991;107(5):46-9. 32.Mapstone R. Corneal thermal patterns in anterior uveitis. Br J Ophthalmol. 1968 Dec;52(12):917-21. 33.Safronenkova IA. [Diagnostic value of orbital skin thermography in unilateral endogenous uveitis]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Odesa: Filatov Institute of Eye Disease and Tissue Therapy; 1989. Russian. 34.Zadorozhnyy O.S., Savin NV, Buiko AS. Improving the technique for controlled cryogenic destruction of conjunctival tumors located in the projection of the ciliary body onto the sclera: A preliminary report. J Ophthalmol (Ukraine). 2018; 5:60-65. 35.Gokul KC, Gurung DB, Adhikary PR. Тhermal effects of eyelid in human eye temperature model. J Appl Math Informatics. 2014; 32 (5-6): 649–63. 36.Rushton JO, Tichy A, Nell B. Introduction of the use of thermography and thermometry in the diagnosis of uveitis in horses: a pilot project. Vet Rec Open. 2015 Jun 27;2(1):e000089.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|