J.ophthalmol.(Ukraine).2020;3:37-41.
|
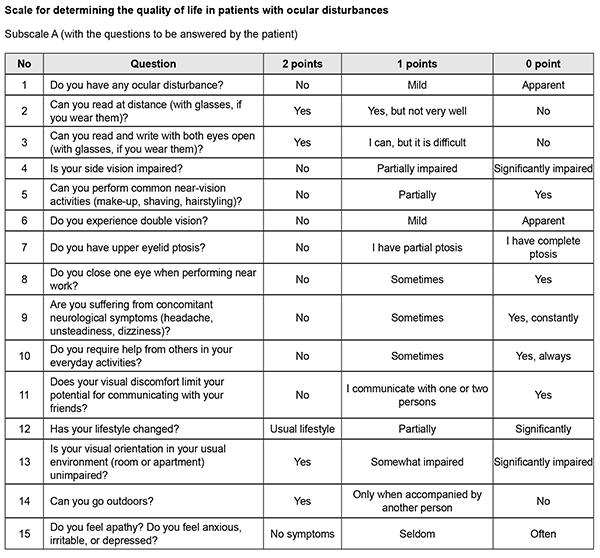
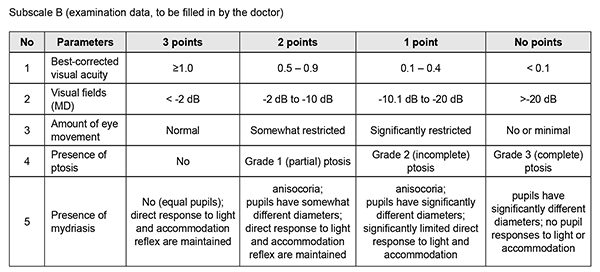
http://doi.org/10.31288/oftalmolzh202033741 Received: 13 March 2020; Published on-line: 24 June 2020 Assessing quality of life in patients with ocular disturbances who underwent surgery for non-functioning pituitary adenoma K.S. Iegorova, O.Ie. Skobska, V.M. Zhdanova, M.O. Guk Romodanov Neurosurgery Institute, NAMS of Ukraine; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net TO CITE THIS ARTICLE: Iegorova K.S., Skobska O.Ie., Zhdanova V.M., Guk M.O. Assessing quality of life in patients with ocular disturbances who underwent surgery for non-functioning pituitary adenoma. J.ophthalmol.(Ukraine).2020;3:37-41. http://doi.org/10.31288/oftalmolzh202033741 Background: Ocular disturbances play a major role in the clinical course of non-functioning pituitary adenoma (NFPA). Partial or complete loss of sight affects patient quality of life (QoL), leading to increased dependence and poor performance; in addition, it affects the patient’s level of social adaptation. Developing a QoL questionnaire for patients with NFPA having ocular disturbances will allow for longitudinal monitoring of a patient’s condition and assessing quality of provided care. Purpose: To develop and implement a method for assessing quality of life in patients with ocular disturbances who underwent surgery for non-functioning pituitary adenoma. Material and Methods: We retrospectively reviewed the results of diagnostic studies and treatment outcomes among 100 patients with NFPA having ocular disturbances who received treatment at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2018. Patients underwent clinical-and-neurological, ophthalmological, otoneurological and neuroimaging examinations. We developed ocular-disturbance score assessment method and QoL scale for patients with ocular disturbances involving a number of indices related to ophthalmological symptoms, as well as to physical, psychic and social status of specific patients. Results: The QoL scale has two subscales, subscale A (15 questions with each question having three possible responses) and subscale B (5 questions with each question having four possible responses), with the questions answered by the patient and the doctor, respectively. With the testing completed, total scores are calculated. A total QOL score of 0–15 is considered a poor (or low) QoL; 16–30, a moderate (or good) QoL; and 31–45, a high QoL. Treatment outcome monitoring and assessment of QoL over time for patients with NFPA having ocular disturbances can be performed by comparison of total pre-treatment and post-treatment scores. Not only is the extent of injury to the optic and oculomotor nerves assessed, but also the impact of physical handicap on the patient’s activities and on his or her functional abilities. Conclusion: Implementing of the method for assessing the quality of life in patients with ocular disturbances with the help of the scale will allow for longitudinal condition monitoring in the course of treatment for patients with NFPA, making it possible to objectify treatment outcomes. Keywords: non-functioning pituitary adenoma, ocular disturbances, oculomotor disturbances, quality of life scale
Introduction Pituitary adenoma (PA) is a neoplasm that develops from anterior pituitary cells and accounts for 20-25% of all intracranial extracerebral tumors. It has been reported that 40-65% of patients with non-functioning PA (NFPA) have ocular disturbances. Specifically, reduced visual acuity and visual field impairment have been found in 38-68.5% and 68-70%, respectively, of patients with PA. 30-62% of patients with PA manifest ocular disturbances [1, 2, 3, 4]. Ocular motility disorders (OMD) are common in PA with CS invasion, occurring in 1.4% to 17% of patients with a conventional disease course and in 45% to 57% of patients with pituitary apoplexy [5, 6, 7]. Paralytical strabismus is accompanied by diplopia, dizziness, headache, nausea and instability of gait. Oculomotor nerve (CN III) injury is more common than those of CN IV and/or VI, and is evident by ptosis, mydriasis, and limited upward, downward and inward movements of the globe. Prolonged compression of the chiasm results in the development of optic nerve atrophy in 31% to 72% of patients, leading to blindness in 3.5% to 16% of patients [8, 9]. In 1948, the World Health Organization defined health from a new perspective, stating that health was defined not only by the absence of disease and infirmity, but also by the presence of physical, mental and social well-being. It was the literature on health status measures that introduced the term “health-related quality of life” (HRQoL) in the second half of the last century [10]. Partial or complete loss of sight affects patient quality of life (QoL), leading to increased dependence and poor performance; in addition, it affects the patient’s level of social adaptation. Not only early diagnosis and surgical treatment for NFPA are important, but also maintaining and improving QoL for these patients. QoL is a multifaceted measure that best reflects the prospects of the patient and his/her caregivers for success [11]. It is a multidimensional construct that focuses on physical, psychical and social aspects, the presence and severity of disease symptoms, taking in account treatment outcomes [12]. Common QoL instruments are tests, questionnaires, or scales. A large number of questionnaires (generic (SF-36, SIP, EQ5D), vision-related (VF-14, NEI-VFQ, NEI-VFQ-25, ADVS) and glaucoma-specific (GSS, COMTOL, GQL-15, SIG)) are available for assessing QoL in patients with ophthalmological manifestations. The most popular among vision-specific questionnaires are NEI-VFQ and NEI-VFQ-25 [13, 15, 16]. The National Eye Institute Visual Function Questionnaire (NEI-VFQ) is widely used for assessing QoL in patients with central retinal dystrophy, diabetic retinopathy, glaucoma and cataract. The original 51-item questionnaire comprises 12 subscales. The patient completes questions, and responses are scored on a scale. However, because that version was too long and required too much time to complete, the 25-item version (NEI VFQ–25) was designed. It comprised the 12 subscales related to general health; general vision; near, distance, peripheral and color vision; social functioning; mental health; vision-specific role difficulties, dependency, ocular pain and driving [13, 14, 15]. There are subjective (physician’s assessment) and objective (self-assessment) approaches to assessing QoL, but using an integrated approach seems to be most reasonable. Self-assessed QoL is a valuable and reliable index of patient’s condition, and, together with a clinical appraisal by a physician, allows for creating a holistic picture of disease course. Problems in determining loss of quality of life develop in 70% of cases. Therefore, in order to objectively assess quantitative and qualitative criteria of patient’s quality of life, and subsequently predict the dynamics of social adaptation and working capacity, it is reasonable to perform a comprehensive clinical and instrumental examination of the patient. Developing a QoL questionnaire for patients with NFPA having ocular disturbances will allow for longitudinal monitoring of a patient’s condition and assessing quality of provided care. The purpose of the study was to develop and implement a method for assessing quality of life in patients with ocular disturbances who underwent surgery for non-functioning pituitary adenoma. Material and Methods We retrospectively reviewed the results of diagnostic studies and treatment outcomes among 100 patients with NFPA having ocular disturbances (42 women and 58 men; aged 24 to 76 years; mean age, 55.5 ± 2.07 years) who received treatment at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2018. All patients underwent transsphenoidal pituitary adenoma resection due to ocular indications. In addition, clinical-and-neurological, ophthalmological, and otoneurological (a routine otorhinolaryngological examination with assessment of cranial nerve function) examinations were performed. Neuroimaging included sella turcica X-ray study with AXIOM Iconos R100 (Siemens) or Radrex-I (Toshiba) in 72 patients, magnetic resonance imaging (MRI) of the brain with a 1.5-T MRI system (Intera 1.5T/I system, Philips Medical Systems, Best, the Netherlands) in all patients, and computed tomography (CT). The MRI of brain and pituitary gland were obtained using T1-weighted image (WI) and T2WI. Neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, static automated perimetry (Centerfield 2 Perimeter, Oculus, Wetzlar, Germany), and direct and indirect ophthalmoscopy. In this study, the degree of ptosis was graded as mild (or grade 1; 1-3 mm lower-than-normal position of the upper lid margin), moderate (or grade 2; the upper eyelid covers half of the pupil) or severe (or grade 3; the upper eyelid covers the pupil completely). The amount of eye movement (as per Golovin) was defined as normal (37° upward, 53° downward, 43° outward, or 46° inward), slightly restricted (19° to 36° upward, 26° to 52° downward, 21° to 42° outward, or 21° to 45° inward), significantly restricted (6° to 20° outward, 6° to 20° inward, 6° to 18° upward, or 27° to 52° downward), or no or almost no (0° to 5°) eye movement. We developed ocular-disturbance score assessment method and QoL scale involving a number of indices related to ophthalmological symptoms, as well as to physical, psychic and social status of patients. Rehabilitation activities profile (Van Bennecom et al, 1995) and NEI VFQ–25 (Mangione et al, 2001) were used as prototypes in developing the scale. This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Romodanov Institute. Written informed consent was obtained from all individuals enrolled in the study. The data were statistically processed using Statistica 6.0 (StatSoft, Tulsa, OK). The results are presented as the mean and standard deviation (M ± SD). Results The QoL scale has two subscales, subscale A (15 questions with each question having three possible responses) and subscale B (5 questions with each question having four possible responses), with the questions answered by the patient and the doctor, respectively. As per WHO guidelines, the patient’s status assessment is based on not only the intensity of pathological process but also influence of the disease or of the trauma on the patient’s self-care ability, home and social activities [10]. Comparison of physician's assessment scores with patient’s self-assessment scores makes it possible to deepen understanding of the functional defect and of the patient’s adaptation to this condition. With the testing completed, total scores are calculated. A total QOL score of 0–15 is considered a poor (or low) QoL; 16–30, a moderate (or good) QoL; and 31–45, a high QoL. Treatment outcome monitoring and assessment of QoL over time for patients with NFPA having ocular disturbances can be performed by comparison of total pre-treatment and post-treatment scores. Not only is the extent of injury to the optic and oculomotor nerves assessed, but also the impact of physical handicap on the patient’s activities and on his or her functional abilities. Clinical example A man, aged 45 years, was hospitalized for pituitary adenoma with signs of pituitary apoplexy. At presentation, visual acuity was mildly decreased; there were bitemporal visual field defects, right oculomotor and abducens neuropathy, mild ptosis, limited eye movements in all directions, and esotropia. The patient’s baseline total QOL score was 10, reflecting “poor” (or low) QoL. The patient responded well to resection of the tumor, with improvements in diplopia and peripheral vision over time. He was re-examined, his QoL was re-assessed with the QoL Scale, and the total QOL score was 24, reflecting “good” (or moderate) QoL. The patient was discharged from the inpatient unit with recommendations for follow-up at two months. At 2 months, visual acuity restored to 1.0, peripheral vision was still somewhat limited, and eye movements (upward, downward, and inward) were completely restored. The patient was re-examined, and his QoL was re-assessed with the QoL Scale. His total QOL score was 41, reflecting “high” QoL. The method for assessing the QoL in patients with ocular disturbances has a number of benefits like no requirement for pre-training; easy and understandable questions can be completed in less than 5 minutes; easy score computation; and integration of subjective and objective approaches.
Conclusion The method for assessing the quality of life in patients with ocular disturbances with the help of the scale should be integrated into the patient examination system involving objective techniques and allows for longitudinal condition monitoring in the course of treatment for patients with NFPA. References 1.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015; 76(3):210-9. DOI:10.1007 / s11102-019-00960-0.Crossref PubMed 2.Lee IH, Miller NR, Zan E, Tavares F, Blitz A, Sung H, et al. Visual Defects in Patients With Pituitary Adenomas: The Myth of Bitemporal Hemianopsia. AJR Am J Roentgenol. 2015 Nov;205(5): W512-8.Crossref PubMed 3.Kan E, Kan EK, Atmaca A, Atmaca H, Colak R. Visual field defects in 23 acromegalic patients. Int Ophthalmol. 2013 Oct;33(5):521-5.Crossref PubMed 4.Ogra S, Nichols AD, Stylli S, Kaye AN, Savino PJ, Danesh-Meyrr HV. Visual acuity and pattern of visual field loss at presentation in pituitary adenoma. J Clin Neurosci. 2014; 21(5):735-40.Crossref PubMed 5.Kim SH, Lee KC, Kim SH. Cranial nerve palsies accompanying pituitary tumour. J of Clinical Neurosci. 2007 Dec;14(12): 1158-62.Crossref PubMed 6.Chuang CC, Chen E, Huang YC, Tu PH, Chen YL, Pai PC. Surgical outcome of oculomotor nerve palsy in pituitary adenoma. J Clin Neurosci. 2011 Nov;18(11):1463-8.Crossref PubMed 7.Hage R, Eshraghi SR, Oyesiku NM, Ioachimescu AG, Newman NJ, Biousse V, et al. Third, fourth, and sixth cranial nerve palsies in pituitary apoplexy. World Neurosurg. 2016 Oct;94:447-452.Crossref PubMed 8.Nishimura M, Kurimoto T, Yamagata Y, Ikemoto H, Arita N, Mimura O. Giant pituitary adenoma manifesting as homonymous hemianopia. Jpn J Ophthalmol. 2007 Mar-Apr;51(2):151-3.Crossref PubMed 9.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 10.World Health Organization. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 2005; 41(10):1403-9.Crossref 11.Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary. 2003 Sep;6(2):81-7.Crossref PubMed 12.Aaronson NK. Quality of life: what is it? How should it be measured? Oncology (Williston Park). 1988 May;2(5):69-76, 64. 13.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-Item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119(7):1050-8.Crossref PubMed 14.Wolf A, Goncalves S, Salehi F, Bird J, Cooper P, Van Uum S, et al. Quantitative evaluation of headache severity before and after endoscopic transsphenoidal surgery for pituitary adenoma. J Neurosurg. 2017; 127(2):409-16.Crossref PubMed 15.Makarenko OV, Kryvoviaz OV, Kryvoviaz SO. [Assessment of the quality of life in patients with primary open-angle glaucoma and approaches to rational pharmacotherapy in this pathology]. Ratsionalna farmakoterapiia. 2016; 2(39): 32-40. Ukrainian. 16.Muslimova ZR. [Rehabilitation treatment of patients with ocular disturbances after removal of tumors of the chiasm and sellar region]. Thesis for the degree of Cand Sc (Med). St Petersburg; 2017. Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|