J.ophthalmol.(Ukraine).2020;3:23-28.
|
http://doi.org/10.31288/oftalmolzh202032328 Received: 20 February 2020; Published on-line: 24 June 2020 ICAM-1 expression on blood lymphocytes in patients with stromal herpes keratitis at different periods of disease N.I. Khramenko, T.B. Gaidamaka, G.I. Drozhzhyna, L.N. Velychko, A.V. Bogdanova SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Odesa (Ukraine) E-mail: khramenkon@gmail.com TO CITE THIS ARTICLE: Khramenko N.I., Gaidamaka T.B., Drozhzhyna G.I., Velychko L.N., Bogdanova A.V. ICAM-1 expression on blood lymphocytes in patients with stromal herpes keratitis at different periods of disease. J.ophthalmol.(Ukraine).2020;3:23-28. http://doi.org/10.31288/oftalmolzh202032328 Background: In spite of intensive antiviral and anti-inflammatory therapy, recovery is not always achieved in patients with stromal herpes keratitis (HK). Inflammatory host response and a complex immune defense system play a major role among the numerous factors involved in herpes simplex virus (HSV) reactivation from latency. Inter-Cellular Adhesion Molecule (ICAM-1) is best known for its role in mediating leukocyte adhesion to endothelial cells and guiding leukocytes across the vascular wall. This factor is considered as an inflammatoty biomarker. Given that signs of inflammation in recurrent stromal keratitis are not always clinically manifest, additional diagnostics is required for well-founded anti-inflammatory treatment decisions. Purpose: To assess the expression of ICAM-1 on blood lymphocytes in patients with primary stromal HK and patients experiencing one or more annual recurrences of stromal HK at different periods of disease. Material and Methods: Fifty-six patients (age, 20 to 60 years) who had been consulted and treated for stromal HK at the Department of Corneal Disorders and 27 systemically and ophthalmologically healthy controls (age, 20 to 64 years) were involved in the study. Of the 56 patients, 10 had primary HK with disease duration ? 6 months, and 46 had recurrent HK with disease duration ? 20 years. Individuals experiencing more than one recurrence annually were defined as those with frequent recurrences. Absolute and relative ICAM-1 expression on lymphocytes was examined. Immunohistochemical staining with monoclonal antibodies for ICAM-1 (CD-54) was performed. Results: The median and interquartile range of the absolute expression level of ICAM, and the mean (standard deviation) of the relative ICAM-1 expression on peripheral blood lymphocytes in controls were 113.3 cells/?L, 87-168 cells/?L, and 8.5 (2.0)%, respectively. Total patients with HSV keratitis exhibited significantly (4.0 times; p = 0.0001) higher absolute counts of peripheral blood lymphocytes expressing ICAM-1 (median, 450 cells/?L; interquartile range, 326-552 cells/?L) compared to controls. In patients in remission with recurrent stromal HK, the relative ICAM-1 expression did not depend on the frequency of recurrence and was 23.4 (4.0)%, which was 2.8 times higher than in controls (p = 0.000). Patients with active keratitis experiencing one recurrence annually had practically the same relative ICAM-1 expression as those experiencing more than one recurrence annually, with a mean value of 26.8 (4.6)%. This was 3.3 times higher than in controls (p = 0.000) and 15.8% higher than in patients in remission with recurrent HK (23.4 (4.1)%; p = 0.001). The relative ICAM-1 expression in patients with primary keratitis was 16% higher than in patients on remission, and there was no significant difference in this parameter between patients with primary keratitis and patients with active recurrent HK. Conclusion: The absolute and relative ICAM-1 expression on peripheral blood lymphocytes in patients with HSV keratitis was 4.0 times and 3.1 times, respectively, higher than in controls. The relative expression of ICAM on lymphocytes in patients with active recurrent disease and in patients with primary keratitis was 16% higher than in patients on remission. Keywords: recurrent herpes keratitis, ICAM-1, lymphocytes
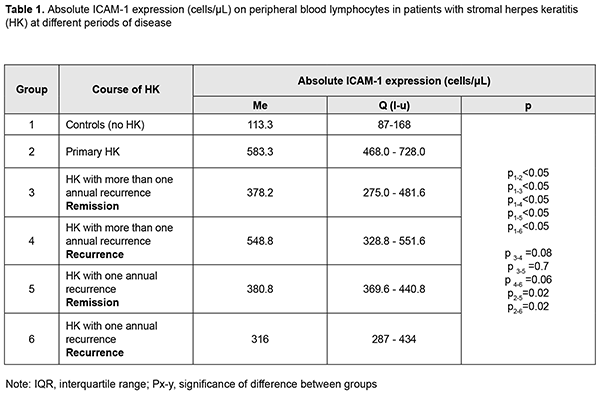
Introduction Inter-Cellular Adhesion Molecule (ICAM-1) or CD54, a member of the immunoglobulin supergene family with five immunoglobulin-like domains, is expressed on cell surface of leukocytes, keratinocytes, and endothelial and epithelial cells, and regulates leukocyte function and migration to sites of inflammation [1]. In addition, it is considered as a functional biomarker of inflammation. ICAM-1 interactions with the beta2 integrins CD11a/CD18 (LFA-1) and CD11b/CD18 (MAC-1) on the surface of leukocytes are important for their transendothelial migration to sites of inflammation and their function as costimulatory molecules for T cell activation. ICAM-1 is constitutively expressed on the cell surface and is up-regulated in response to a variety of inflammatory mediators, including proinflammatory cytokines, hormones, cellular stresses, and virus infection [2]. LFA-1 binds with high affinity to ICAM-1, its major ligand, and with lower affinity to ICAM-2 or ICAM-3. ICAM-1 binding to LFA-1 results in signaling to the interior of the cell, with aggregation of some of the key molecules on the plasma membrane. ICAM-1 is important for migration of lymphocytes from blood across the vascular endothelium into lymph nodes or inflamed tissues (e.g., the ocular surface). During inflammation, ICAM-1 expression is upregulated by proinflammatory cytokines (like TNF-?) on vascular and lymphatic endothelial cells, as well as other tissue cells. Inflamed lymphatic endothelium promotes the exit of leukocytes from tissue to afferent lymph through newly induced expression of ICAM-1 [3]. ICAM-1 is up-regulated during inflammation by several cell types, including endothelial cells and leukocytes. Recently, macrophages have been shown to express ICAM-1. Tissue macrophages play a critical role in removing apoptotic/necrotic cells in inflammation and injury, a process termed efferocytosis. A role for ICAM-1 in promoting macrophage efferocytosis, a critical process in the resolution of inflammation and restoration of tissue homeostasis, has been defined [4]. In addition, the role that LFA-1/ICAM-1 interaction plays in the afferent and efferent arms of the immunoinflammatory pathway of DED has been described. Inhibition of LFA-1/ICAM-1 interaction represents a rational targeted approach in treating DED. Notably, inhibition of LFA-1/ICAM-1 binding with lifitegrast offers a novel approach to reducing ocular surface inflammation in this condition [1]. Proinflammatory cytokines, such as TNF-?, IL-1, and IFN-?, stimulate endothelial cells to express receptors for intercellular adhesion molecules such as ICAM-1 on white blood cells [5]. IL-10 does not inhibit the expression of ICAM-1, but is inhibited by glucocorticoids [6]. ICAM-1 plays a role in inflammatory processes and in the T-cell mediated host defense system [7]. The role of ICAM-1 in autoimmune disorders, severe cardiovascular events (myocardial infarctions), endocrine disorders (diabetes mellitus, thyroid disorders), psychic disorders, has been reported. ICAM-1 has been chosen as a biomarker of inflammation in viral disorders including herpes simplex virus (HSV). The expression of ICAM-1 is increased in viral encephalitis caused by HSV. Viral encephalitis is characterised by lymphocytic infiltration of the CNS and one mechanism of this response may be EC adhesion molecule induction with consequent inflammatory cell/EC binding [8]. A study by Zhang et al [9] aimed to elucidate the role of adhesion molecules in the pathogenesis of herpes simplex keratitis. The corneas of mice infected with HSV were examined immunohistochemically at days 14 and 21 after infection. ICAM-1 was mainly expressed in the basal cells of the corneal epithelia and vascular endothelia of the infected mice. The authors concluded that ICAM-1 molecules were involved in the progression of HSV and may accelerate the progress of inflammation by mediating the extravasation of inflammatory cells from vessels into the infected sites [9]. In a study by Elner et al [10], corneal specimens of patients with stromal HSV keratitis were examined immunohistochemically. These specimens demonstrated intense ICAM-1 immunoreactivity of keratinocytes, stromal keratocytes, and endothelial cells, predominantly in regions of leukocytic infiltration. The authors concluded that increased ICAM-1 expression in regions of leukocytic infiltration may regulate leukocyte-corneal cell binding, thereby promoting immune responses and damage by activated leukocytes [10]. Stromal HSV keratitis in humans is characterized by infiltration of inflammatory cells including lymphocytes, neutrophils, and mononuclear phagocytes. In a study by Shtein et al [11], corneal specimens demonstrating inflammation had significantly increased IL-8 and MCP-1 levels and greater immunoreactivity for HLA-DR and ICAM-1 when compared with specimens without inflammation. Expression of these molecules is known to enhance antigen recognition and subsequent allograft rejection which the authors found to correlate with the presence of inflammation [11]. Treatment with anti-inflammatory IL-10 has been proposed for numerous inflammatory ocular disorders. IL-10 treatment ex vivo, however, significantly inhibited HLA-DR, IL-8, and MCP-but did not reduce ICAM-1 expression [6]. Integration of knowledge concerning membrane-bound and soluble ICAM-1 into a single functional system is likely to contribute to elucidating the immunoregulatory function of ICAM-1 and its pathophysiological significance in various disease entities [7]. To the best of our knowledge, the role of ICAM-1 expression in herpes keratitis and in the pathogenesis of recurrent HK has been poorly studied. Given that signs of inflammation in recurrent stromal keratitis are not always clinically manifest, additional diagnostics is required for well-founded anti-inflammatory treatment decisions. The purpose of this study was to assess the expression of ICAM-1 on blood lymphocytes in patients with primary stromal HK and patients with recurrent stromal HK experiencing frequent or infrequent recurrences at different periods of disease. Material and Methods Fifty-six patients (age, 20 to 60 years; mean age, 42.0±2.0 years) who had been consulted and treated for stromal HK at the Department of Corneal Disorders and 27 systemically and ophthalmologically healthy controls (age, 20 to 64 years) were involved in the study. Of the 56 patients, 10 had primary HK with disease duration ? 6 months, and 46 had recurrent HK with disease duration ? 20 years. Patient visual acuity varied from 0.03 to 1.0, and no patient had concurrent somatic disease. Patients experiencing more than one recurrence annually were defined as those with frequent recurrences. Blood was collected from the medial cubital vein using a disposable vacuum collection tube system. Immunohistochemical staining with monoclonal antibodies for ICAM-1 (CD-54) was performed [12]. Absolute and relative ICAM-1 levels were examined and expressed in cells/?L and percentages, respectively. Median, interquartile range (IQR), and mean and standard deviation (SD) values were calculated. Mann-Whitney, Kruskal-Wallis and Student tests were used for statistical analyses. Results The median and IQR of the absolute expression level of ICAM on peripheral blood lymphocytes in controls were 113.3 cells/?L and 87-168 cells/?L, respectively. It is noteworthy that all the 56 patients with HSV keratitis exhibited significantly higher absolute counts of peripheral blood lymphocytes expressing ICAM-1 (median, 450 cells/?L; IQR, 326-552 cells/?L) compared to controls (Table 1). In 10 patients with primary HK (mean disease duration, 120 days), the median of the expression level of ICAM on peripheral blood lymphocytes was 583.3 cells/?L, which was 5.4 times higher than in controls. In addition, the IQR was 468.0-728.0 cells/?L (Table 1).
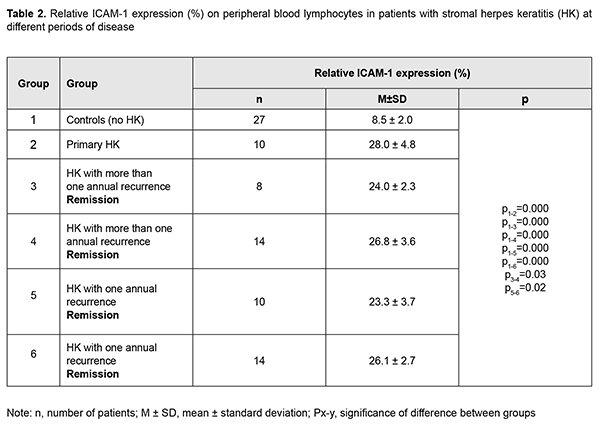
Twenty-two patients experienced more than one recurrence annually. Of these, 8 patients exhibited remission, and 14 exhibited recurrence. The median of the expression level of ICAM on peripheral blood lymphocytes in the former patients was 378.2 cells/?L (i.e., 3.3 times higher than in controls, p < 0.05), and in the latter patients, 548.8 cells/?L (i.e., 4.8 times higher than in controls, p < 0.05). Twenty patients experienced one recurrence annually. In these patients, the median of the absolute expression level of ICAM on peripheral blood lymphocytes was 384.6 cells/?L (i.e., 3.4 times higher than in controls, p < 0.05; IQR, 295.9-450.8 cells/?L), with no significant difference between patients exhibiting remission and those exhibiting recurrence. In addition, there was no significant difference in this parameter between patients on remission experiencing more than one recurrence annually and patients on remission experiencing one recurrence annually. Absolute ICAM-1 level in patients with primary keratitis was significantly higher than in patients on recurrence experiencing one recurrence annually, or in patients on remission experiencing one recurrence annually (p = 0.027). Percentage of lymphoid cells expressing ICAM-1 is a relative ICAM-1 expression on lymphocytes and was also assessed in the study groups. The relative ICAM-1 expression in the total group of patients with HSV keratitis was significantly (3.1 times) higher than in controls (26.1(4.9)% vs 8.5 (2.0)%). In addition, the relative ICAM-1 expression in patients with primary HK was 3.3 times higher than in controls (p = 0.000; Table 2).
In patients in remission with recurrent stromal HK, the relative ICAM-1 expression did not depend on the frequency of recurrence and was 23.4 (4.0)%, which was 2.8 times higher than in controls (p = 0.000). Patients with active keratitis experiencing one recurrence annually had practically the same relative ICAM-1 expression as those experiencing more than one recurrence annually, with a mean value of 26.8 (4.6)%. This was 3.3 times higher than in controls (p = 0.000) and 15.8% higher than in patients in remission with recurrent HK (23.4 (4.1)%; (p = 0.001). The relative ICAM-1 expression in patients with primary keratitis was 16% higher than in patients on remission, and there was no significant difference in this parameter between patients with primary keratitis and patients with active recurrent HK. Therefore, the absolute and relative ICAM-1 expression on peripheral blood lymphocytes in total patients with HSV keratitis was 4.0 times and 3.1 times, respectively, higher than in controls. Particularly, these characteristics in patients with primary keratitis were 5.3 times and 3.3 times, respectively, higher, compared to controls. In addition, in patients with active recurrent disease, they were 4.8 times and 3.3 times, respectively, higher, and in patients in remission with recurrent HSV keratitis, they were 3.4 times and 2.8 times, respectively, higher, compared to controls. The ICAM-1 expression in patients with primary keratitis and in patients with active recurrent disease was 16% higher than in patients in remission with recurrent HSV keratitis (p = 0.007). Hence, the ICAM-1 expression on peripheral blood lymphocytes can be considered as an inflammatory index in patients with HK. Discussion Ocular infection with HSV continues to be a serious clinical problem despite the availability of effective antiviral and anti-inflammatory drugs. After initial ocular infection, HSV can establish latent infection in the trigeminal ganglia for the lifetime of the host. During latency, the viral genome is retained in the neuron without producing viral proteins. Recurrent disease occurs as HSV-1 is carried by anterograde transport to the original site of infection, or any other site innervated by the latently infected ganglia, and can reinfect the ocular tissues. Recurrent corneal disease can lead to corneal scarring, thinning, stromal opacity and neovascularization and, eventually, blindness. In spite of intensive antiviral and anti-inflammatory therapy, a significant percentage of patients do not respond to medicamentous therapy for herpetic stromal keratitis. Inflammatory host response and immune defense system play a major role among the numerous factors involved in HSV reactivation from latency [13, 14, 15]. ICAM-1 is best known for its role in mediating leukocyte adhesion to endothelial cells and guiding leukocytes across the vascular wall. This factor is considered as an inflammatory biomarker [4]. ICAM-1 expression is known to be increased in HSV infection [16]. Experimental studies [17, 18] demonstrated that ICAM-1 had no effect on the time-course of appearance or the intensity of the inflammatory infiltrate, but indirectly contributed to the production of IFN-gamma and improved survival of experimental animals infected with HSV. The present study found that the absolute expression of ICAM on peripheral blood lymphocytes in patients with HSV keratitis was 3.3 times to 5.3 times higher than in controls. In addition, the relative expression of ICAM on lymphocytes in patients with active recurrent disease and in patients with primary keratitis was significantly (16%) higher than in patients on remission. Hence, expression of ICAM on peripheral blood lymphocytes can be considered as an indicator of chronic inflammation and active disease in stromal herpes kerartitis. Conclusion First, the absolute and relative ICAM-1 expression on peripheral blood lymphocytes in total patients with HSV keratitis was 4.0 times and 3.1 times, respectively, higher than in controls. Second, the relative expression of ICAM on lymphocytes in patients with active recurrent disease and in patients with primary keratitis was significantly (16%) higher than in patients on remission. References 1.Pflugfelder SC, Stern M, Zhang S, Shojaei A. LFA-1/ICAM-1 Interaction as a Therapeutic Target in Dry Eye Disease. J Ocul Pharmacol Ther. 2017 Jan/Feb;33(1):5-12. 2.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999 Dec;66(6):876-88. 3.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006 Nov 27;203(12):2763-77. 4.Wiesolek HL, Bui TM, Lee JJ, Dalal P, Finkielsztein A, Batra A, et al. ICAM-1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol. 2020 Apr;190(4):874-85. doi: 10.1016/j.ajpath.2019.12.006. 5.Hocaoglu-Emre FS, Saribal D, Yenmis G, Guvenen G. Vascular Cell Adhesion Molecule 1, Intercellular Adhesion Molecule 1, and Cluster of Differentiation 146 Levels in Patients with Type 2 Diabetes with Complications. Endocrinol Metab (Seoul). 2017 Mar;32(1):99-105. 6.Shtein RM, Garcia DD, Musch DC, Elner VM. HSV keratitis: histopathologic predictors of corneal allograft complications. Trans Am Ophthalmol Soc. 2008;106:161-8; discussion 168-70. 7.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl). 1996 Jan;74(1):13-33. 8.Brankin B, Hart MN, Cosby SL, Fabry Z, Allen IV. Adhesion molecule expression and lymphocyte adhesion to cerebral endothelium: effects of measles virus and herpes simplex 1 virus. J Neuroimmunol. 1995 Jan;56(1):1-8. 9.Zhang T, Wang N, Lou B, Yuan Z. The role of adhesion molecules ICAM-1 and VCAM-1 in herpes simplex keratitis. Eye Sci. 2011 Jun;26(2):61-4. 10.Elner VM, Dutt S, Pavilack MA, Sugar A, Foster CS, Elner SG. Intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigens in herpes keratitis. Ophthalmology. 1992 Sep;99(9):1400-7. 11.Shtein RM, Garcia DD, Musch DC, Elner VM. Herpes simplex virus keratitis: histopathologic inflammation and corneal allograft rejection. Оphthalmology. 2009 Jul;116(7):1301-5. 12.Gluzman DF, Skliarenko LM, Nadgornaia VA, Kriachok IA. [Immunocytochemistry in tumor diagnosis]. Kyiv: Morion;2003. Russian. 13.Toma HS, Murina AT, Areaux RG Jr, Neumann DM, Bhattacharjee PS, Foster TP, Kaufman HE, Hill JM. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008 Jul-Aug;23(4):249-73. 14.Gaidamaka TB, Chaura AG, Khramenko NI. [State of the autonomous nervous system and psychoemotional status in patients with herpetic keratitis]. Oftalmol Zh. 2009;1-2:70-2. Ukrainian. 15.Khramenko NI, Ponomarchuk VS, Drozhzhyna GI. [State of the autonomous nervous system and effect of the latter on regional ocular hemodynamics in patients with different courses of recurrent herpetic keratitis]. Oftalmol Zh. 2013;6:5-11. Ukrainian. 16.Kim YC, Bang D, Lee S, Lee KH. The effect of herpesvirus infection on the expression of cell adhesion molecules on cultured human dermal microvascular endothelial cells. J Dermatol Sci. 2000 Sep;24(1):38-47. 17.Jung HW, Jung CR, Choi BK, Vinay DS, Hill JM, Gebhardt BM, Kwon BS.Herpesvirus infection of ICAM-1-deficient mice. Curr Eye Res. 2004 Aug-Sep;29(2-3):201-8. 18.Noisakran S, H?rle P, Carr DJ. ICAM-1 is required for resistance to herpes simplex virus type 1 but not interferon-alpha1 transgene efficacy. Virology. 2001 Apr 25;283(1):69-77.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|