J.ophthalmol.(Ukraine).2020;3:16-22.
|
http://doi.org/10.31288/oftalmolzh202031622 Received: 27 April 2020; Published on-line: 24 June 2020 Accelerated CXL for stage 2 to 3 progressive keratoconus: 24-month outcomes L.F. Troichenko, G.I. Drozhzhyna SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Одеса (Україна) E-mail: cornea.@te.net.ua TO CITE THIS ARTICLE: Troichenko L.F., Drozhzhyna G.I. Accelerated CXL for stage 2 to 3 progressive keratoconus: 24-month outcomes. J.ophthalmol.(Ukraine).2020;3:16-22. http://doi.org/10.31288/oftalmolzh202031622 Background: Ultraviolet (UV-X) corneal collagen cross-linking (CXL) involves photopolymerization of corneal collagen and in recent decades has been commonly employed for the treatment of stage 2 to 3 keratoconus. The high-intensity illumination of the UV-X™ 2000 Crosslinking System (Avedro, Inc., Waltham, Massachusetts) provides accelerated CXL (A-CXL), reducing treatment time threefold (to 10 minutes) compared with conventional CXL protocol. Purpose: To assess 24-month outcomes of A-CXL for stage 2 to 3 progressive keratoconus. Material and Methods: One hundred and nineteen patients (167 eyes) who underwent ACXL for keratoconus were included in this study. At 24 months, ocular changes were assessed in 40 eyes. A-CXL was carried out using the UV-X™ 2000 Crosslinking System at an irradiation intensity of 9 mW/cm?. Results: Mean astigmatism decreased by 1.15 D to 3.02 ± 1.73 (SD) D (for n=40 eyes) and mean corneal refractive power as assessed by Kmax decreased by 3.4 D to 54.4 ± 6.62 (SD) D (median value, 54.3 D; n=40 eyes) at 24 months compared to baseline values. Mean thinnest local corneal thickness increased by 3.0 ?m (mean value, 462.7± 34.3 (SD) ?m; median value, 455.5 ?m; n=40 eyes; р = 0.009), mean uncorrected visual acuity increased by 0.2, and mean best-corrected visual acuity increased by 0.25 at 24 months compared to baseline values. Of the 40 eyes examined at 24 months, BCVA improved in 34 eyes (85%). Conclusion: A-CXL for stage 2 to 3 progressive keratoconus resulted in a steady state of the pathological process, decrease in astigmatism by 1.15 D, decrease in corneal refractive power as assessed by Kmax by 3.4 D, and an increase in thinnest local corneal thickness by 3.0 ?m for the total study cohort, and improvement in BCVA in 85% of eyes at 24 months. Keywords: keratoconus, cornea, cross-linking
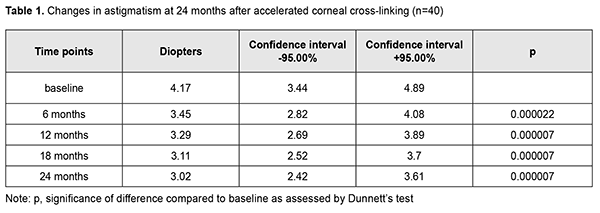
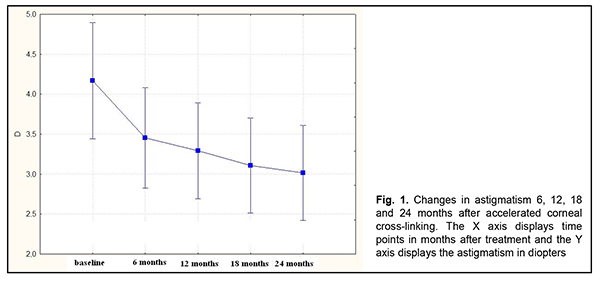
Introduction Progressive degeneration of the collagen structure of the cornea underlies keratoconus and impairs corneal mechanical strength properties, resulting in corneal bulging, opacification and frequently scarring and substantially decreased vision [1-6]. The disease most commonly affects both eyes and is most commonly found in adolescents [7, 8]. In addition, in Ukraine, it is the second most common indication for keratoplasty. A study from the UK found that the incidence of keratoconus was 4.4 times higher in Asians than in white patients [9, 10]. The etiology is considered multifactorial with genetic, endocrine, allergic and immune influences, but genetic influence is most widely recognized [11]. Of the patients enrolled at 16 clinical centers in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study, 13.5% reported a family history [12]. Ultraviolet (UV-X) corneal collagen cross-linking (CXL) involves photopolymerization of corneal collagen and in recent decades has been commonly employed for the treatment of stage 2 to 3 keratoconus. Photochemical ionization takes place and riboflavin is destroyed (with release of free oxygen) by exposure to UV-A radiation generated by the UV-X system. Free oxygen-derived radicals cause cross-linking between -СН and –CN groups in collagen molecules, which induces their binding to form a 3D meshwork. Numerous additional bounds between corneal collagen fibers result in a significant improvement of corneal mechanical strength and rigidity. Biomechanical studies have shown that corneal rigidity increases by 350%-380% after cross-linking [13-26]. Several corneal CXL protocols are available, including an epithelial-off standard protocol of 3 mW/cm2 intensity at 370 nm over an exposure time of 30 minutes (now termed the ‘‘Dresden protocol’’); accelerated CXL protocols carried out in a shorter period such as 3, 5, or 10 minutes by using 30, 18, or 9 mW/cm2 irradiance; and transepithelial CXL protocol (without corneal de-epithelization) [13, 17, 27]. The high-intensity illumination of the UV-X™ 2000 Crosslinking System (Avedro, Inc., Waltham, Massachusetts) provides accelerated CXL, reducing treatment time threefold (to 10 minutes) compared with conventional CXL protocol [13]. Recent studies by Sadoughi [28] and Shajari [29] found that accelerated CXL with 9 mW/cm2 irradiation for 10 min had similar refractive, visual, keratometric, and aberrometric results and less adverse effects on the corneal thickness and endothelial cells as compared with the conventional method after 12 months follow-up. Those studies, however, concluded that clinical trials with longer follow-ups were needed. We have previously reported on 6-month and 12-month treatment outcomes for keratoconus patients treated with CXL [4]. The purpose of the current study was to assess 24-month outcomes of accelerated CXL for stage 2 to 3 progressive keratoconus. Material and Methods One hundred and nineteen patients (167 eyes; 90 men and 29 women) aged 12-57 years (25.3 ± 8.55 SD years; median value, 25 years) who underwent A-CXL for keratoconus were included in this study. Of these eyes, 77 (46.1%) had stage 2 keratoconus, and 90 (53.9 %) had stage 3 keratoconus (Amsler-Krumeich classification). Stage II keratoconus and stage 3 keratoconus were uniformly distributed among men and women (46% and 54%, respectively). In addition, of the 167 eyes, 28 (16.8%) were involved in a unilateral process, and 139 (83.2%) were involved in a bilateral process. Bilateral cross-linking was conducted in 48 (35%) of the 139 eyes. Fifteen fellow eyes required keratoplasty for stage 3 keratoconus. Disease duration until the CXL treatment ranged from one year to 20 years, with a mean value of (3 ± 2.65 SD) years. The disease was diagnosed within 2 years from the onset in 55.1% of cases. Male patients were significantly more frequently diagnosed within five years from the onset of the disease, whereas female patients were significantly more frequently diagnosed within two years from the onset of the disease (?? = 28.3, р = 0.0004). Family history of refractive errors (myopia and/or astigmatism) and association of occurrence of keratoconus progression with stress were reported by 46.2% and 44.6%, respectively, of male patients, and 53.8% and 19.2%, respectively, of female patients. In addition, 26.9% of female patients reported association of occurrence of keratoconus progression with delivery. Patients underwent biomicroscopy, refractometry, and corneal confocal biomicroscopy (Confoscan 4, NIDEK Co., Ltd., Aichi, Japan) in addition to a routine eye examination. Pentacam® apparatus (Oculus Inc., Wetzlar, Germany) was used to perform keratography and corneal pachymetry and to estimate corneal refractive power. Kmax was used as a measure of corneal refractive power, and thinnest local corneal thickness was measured. Uncorrected (UCVA) and best-corrected (BCVA) visual acuities were assessed using the Shevalev chart and the Sivtsev chart and categorized as 1 (VA ? 0.1), 2 (VA ? 0.3), and 3 (VA > 0.3). Studies were performed preoperatively and at 6, 12, 18 and 24 months postoperatively. Studies at months 6, 12, 18 and 24 involved 167 eyes, 149 eyes, 65 eyes, and 40 eyes, respectively. Accelerated CXL was carried out using the UV-X™ 2000 Crosslinking System at an irradiation intensity of 9 mW/cm?. Corneal epithelium was debrided using a diameter of 7.0, 7.5, or 8.0 mm according to corneal topography data. Intraoperative corneal pachymetry was performed thereafter and after the cornea was soaked with riboflavin solution for 15 minutes. The duration of CXL UVA laser exposure was 10 minutes. At the end of procedure, a bandage soft contact lens was placed on the eye. Antiseptics, agents promoting corneal regeneration, preservative-free tear substitutes and (if indicated) antibiotics and antiviral agents were prescribed postoperatively. The bandage soft contact lens was removed after re-epithelization was complete. Mean, error of mean, median, quartile, standard deviation (SD), quartile, and variation range values were calculated. The Shapiro–Wilk and Kolmogorov–Smirnov tests were performed to determine normality of data distribution. Comparison between groups was made using the Student's t test with Levene's test for homogeneity of variance or the Mann-Whitney U test. A one-way ANOVA (F-distribution) or Friedman chi-square test were used to compare characteristics measured under three or more different sets of conditions for the same sample of patients. Kendall concordance test was used to assess the strength of association between variables. Statistical analyses were conducted using Statistica 9.0 (StatSoft, Tulsa, OK, USA) software. Results Complete corneal re-epithelization was noted 3 to 5 days (mean value, 3.8 ± 0.73 SD days) after CXL. At day 3, day 4 and day 5, complete corneal re-epithelization was noted in 44 eyes (26.3 %), 80 eyes (48 %) and 43 eyes (26 %), respectively. The bandage soft contact lens was removed after re-epithelization was complete. No intra-operative complication was noted in any patient. Mean astigmatism decreased from 4.16 ± 2.11(SD) D (for n=167 eyes) at baseline to 3.91 ± 2.34 (SD) D (for n=167 eyes) at 6 months, and 3.79 ± 2.56 (SD) D (for n=149 eyes) at 12 months. In addition, compared to baseline values, it decreased by 1.15 D to 3.02 ± 1.73 (SD) D (for n=40 eyes) at 24 months (?? = 74.3, р = 0.000) (Table 1 and Fig. 1).
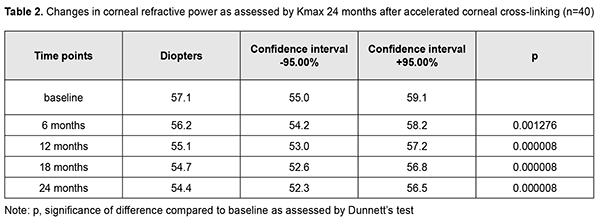
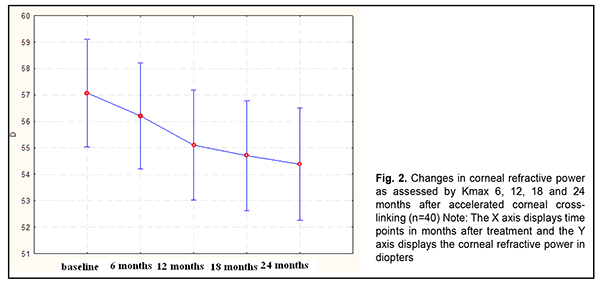
Mean corneal refractive power as assessed by Kmax decreased from 57.8 ± 6.83 (SD) D (range, 45.2 to 68.7 D; median value, 57.9 D; n=167 eyes) at baseline by 1.0 D to 57.0 ± 6.91 (SD) D (median value, 57.0 D; n=167 eyes) at 6 months, and by 1.9 D to 55.9 ± 6.93 (SD) D (median value, 55.0 D; n=149 eyes) at 12 months (р = 0.000). In addition, it did not change significantly (mean value, 55.9± 7.19 (SD) D; median value, 55.8 D; n=65 eyes) at 18 months compared to 12-month values, and decreased by 3.4 D to 54.4 ± 6.62 (SD) D (median value, 54.3 D; n=40 eyes) at 24 months compared to baseline values (р = 0.000) (Table 2 and Fig. 2).
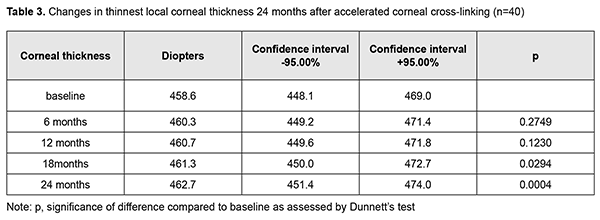
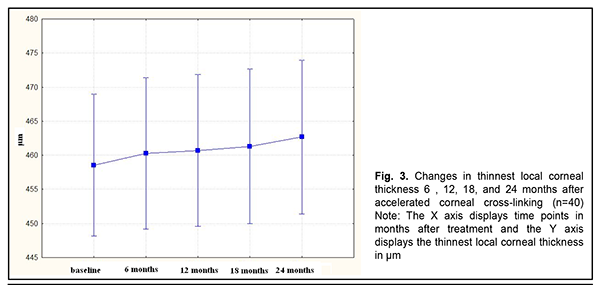
Mean thinnest local corneal thickness was 459.7 ± 36.6 (SD) ?m (median value, 454 ?m; n=167 eyes) at baseline, and did not change significantly (mean value, 465.5± 37.1 (SD) ?m; median value, 450.0 ?m; n=167 eyes) at 6 months. In addition, compared to baseline values, there was no significant change in thinnest local corneal thickness at 12 months (mean value, 453.7± 55.5 (SD) ?m; median value, 455.0 ?m; n=149 eyes; р = 0.48) and 18 months (mean value, 455.1± 35.0 (SD) ?m; median value, 455.0 ?m; n=65 eyes; р = 0.6). There was, however, a significant increase in thinnest local corneal thickness by 3.0 ?m (mean value, 462.7± 34.3 (SD) ?m; median value, 455.5 ?m; n=40 eyes; р = 0.009) at 24 months compared to baseline values (Table 3 and Fig. 3).
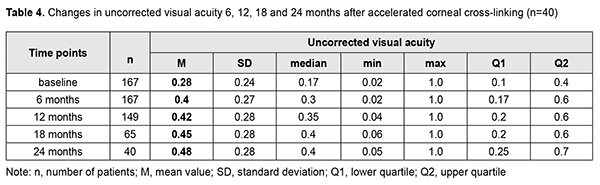
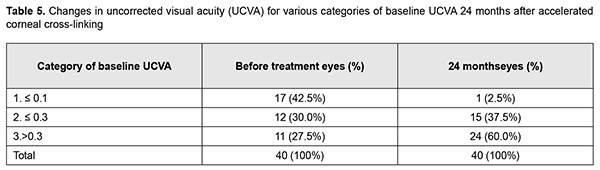
Mean UCVA increased by 0.12 at 6 months, 0.14 at 12 months, 0.17 at 18 months, and 0.2 at 24 months (Table 4) compared to baseline values. In addition, of the 40 eyes examined at 24 months, UCVA improved in 25 eyes (62.5% (CI, 49.2%-75.3%)). Table 5 presents changes in UCVA at 24 months compared to baseline values for eyes relevant to various visual acuity categories.
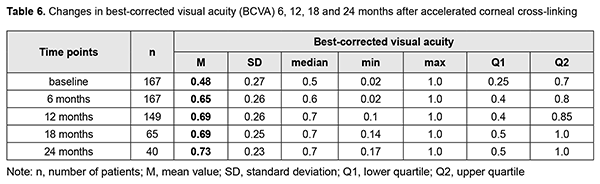
Mean BCVA increased by 0.17 at 6 months, 0.21 at 12 months and 18 months, and 0.25 at 24 months after accelerated CXL compared to baseline values (Table 6). In addition, of the 40 eyes examined at 24 months, BCVA improved in 34 eyes (85% (CI, 71.5%-91.7%)). Moreover, the portion of eyes with vision correctable by spectacles increased by 6%, from 79% to 85%. The number of eyes with vision correctable by spectacles was 132/167 (95% CI, 72.1 – 84.5%) at baseline and 34/40 (95% CI, 71.5 – 91.7%) at 24 months.
Table 7 presents changes in BCVA at 24 months compared to baseline values for eyes relevant to various visual acuity categories.
In 3 eyes, sterile corneal infiltrates were observed postoperatively; they were noted to resolve completely at day 7 to 10. Discussion Therefore, by 24 months after A-CXL for stage 2 to 3 progressive keratoconus, a steady state of the pathological process was achieved in all cases. Keratoconus stabilization was accompanied by a decrease of astigmatism by 1.15 D and of the corneal refractive power by 3.4 D; and recovery of corneal thickness (453.7 ± 55.5(SD) ?m) at 18 months and increase in corneal thickness by 3.0 ?m to 462.7 ± 34.3 (SD) ?m at 24 months compared to baseline values. In addition, UCVA and BCVA improved in 62.3% and 85% of cases, respectively, and confocal microscopy found that recovery of normal corneal architectonics was complete at 12 months and maintained at the last follow-up 24 months after CXL. Our findings are in agreement with those from a recent study by German and Austrian ophthalmologists [28] who conducted a meta-analysis to compare the results of standard and accelerated corneal cross-linking with the follow-up not exceeding 18 months. They concluded that consideration of less corneal thinning favours A-CXL, whereas the deeper demarcation line and greater changes in minimum keratometric values in C-CXL may indicate a higher treatment efficacy. Altogether, C-CXL, as well as A-CXL, provides successful results in the strengthening of corneal tissue. In the current study, 90% of patients reported improved quality of vision and improved spectacle tolerance. Conclusion Accelerated CXL for stage 2 to 3 progressive keratoconus resulted in a steady state of the pathological process, decrease in astigmatism by 1.15 D, decrease in corneal refractive power as assessed by Kmax by 3.4 D, and an increase in thinnest local corneal thickness by 3.0 ?m for the total study cohort, and improvement in BCVA in 85% of eyes at 24 months.
References 1.Bikbov MM, Surkova VK. [Corneal collagen crosslinking for keratoconus]. A review. Ophthalmology in Russia. 2014;11(3):13-8. https://doi.org/10.18008/1816-5095-2014-3-13-19. Russian. 2.Birich TA, Chekina AIu, Aksionova NI. [Outcomes of treatment for keratoconus]. Oftalmologiia Belorusi. 2010;1(4):90-7. Russian. 3.Ivanovskaia EV, Vit VV, Golovchenko VG. [Immunological status of patients with various stages of keratoconus and keratoglobus]. Oftalmol Zh. 2000;5:40-4. Russian. 4.Drozhzhyna GI, Troychenko LF, Naumenko VA, et al. [Outcomes of accelerated corneal collagen cross-linking in keratoconus]. Oftalmol Zh. 2018;3:10-7. Russian. 5.Sevastiianov EN, Gorskova EN, Ekgard VF. [Keratoconus (etiology, pathogenesis, medicinal treatment): textbook]. Cheliabinsk: UGMADO;2005. Russian. 6.Solodkova EG, Remesnikov IA. [Modern approaches in the treatment progressive keratectasia]. Prakticheskaia meditsina. 2012;4:75-9. Russian. 7.Croghale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61(8): 382-3. 8.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998 Jan-Feb;42(4):297-319. 9.Georgiou T, Funnell CL, Cassels-Brown A, O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond). 2004 Apr;18(4):379-83. 10.Li X, Rabinowitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus. Ophthalmology. 2004;111:440–6. 11.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. Gordon-Shaag A, et al. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. 12.Zadnik K, Barr JT, Edrington TB, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. 13.Adel Alhayek, Pei-Rong Lu. Corneal collagen crosslinking in keratoconus and other eye disease. Int J Ophthalmol. 2015; 8(2): 407–18. doi: 10.3980/j.issn.2222-3959.2015.02.35. 14.Kohlhaas M, Spoerl E, Schilde T, et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006 Feb;32(2):279-83. 15.Nowak DM, Gajecka M. The genetics of keratoconus. Middle East Afr J Ophthalmol. 2011 Jan;18(1):2-6. 16.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA – riboflavin cross – linking of the cornea. Cornea. 2007 May;26(4):385-9. 17.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin/ultraviolet–A-induced cross-linking. J Cataract Refract Surg. 2003 Sep;29(9):1780-5. 18.Kymionis GD, Portaliou DM, Bouzoukis DI, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract and Refract Surg. 2007 Nov;33(11):1982-4. 19.Macsai MS, Varley GA, Krachmer JH. Development of keratoconus after contact lens wear. Patient characteristics. Arch Ophthalmol. 1990 Apr;108(4):534-8. 20.Mazzotta C, Balestrazzi A, Traversi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen; ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007 May;26(4):390-7. 21.Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus. Invest Ophthalmol Vis Sci. 2005. 2005 Jun;46(6):1948-56. 22.McQuaid R, Cummings AB, Mrochen M. The theory and art of corneal cross-linking. Indian J Ophthalmol. 2013 Aug;61(8):416-9. 23.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998 Jan;66(1):97-103. 24.Spoerl E, Seiler T J. Techniques for stiffening the cornea. Refract Surg. 999 Nov-Dec;15(6):711-3. 25.Sp?rl E, Huhle M, Kasper M, Seiler T. [Increased rigidity of the cornea caused by intrastromal crosslinking]. Ophthalmologe. 1997 Dec;94(12):902-6. German. 26.Tsubota K, Mashima Y, Murata H, et al. Corneal epithelium in keratoconus. Cornea. 1995 Jan;14(1):77-83. 27.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. – 2003 May;135(5):620-7. 28.Shajari M, Kolb CM, Agha B, Steinwender G, M?ller M, Herrmann E, et al. Comparison of standard and accelerated corneal cross-linking for the treatment of keratoconus: a meta-analysis. Acta Ophthalmol. 2019 Feb;97(1):e22-e35. 29.Sadoughi MM, Einollahi B, Baradaran-Rafii A, et al. Accelerated versus conventional corneal collagen cross-linking in patients with keratoconus: an intrapatient comparative study. Int Ophthalmol. 2018; 38: 67–74.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|