J.ophthalmol.(Ukraine).2020;3:9-15.
|
http://doi.org/10.31288/oftalmolzh20203915 Received: 31 January 2020; Published on-line: 24 June 2020 Visual evoked potentials in 5 to 8-year-old children with retinopathy of prematurity S.V. Katsan, O.Iu. Terletska, A.O. Adakhovska SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Odesa (Ukraine) E-mail: adakhovskayaa@gmail.com TO CITE THIS ARTICLE: Katsan S.V., Terletska O.Iu., Adakhovska A.O. Visual evoked potentials in 5 to 8 year-old children with retinopathy of prematurity. J.ophthalmol.(Ukraine).2020;3:9-15. http://doi.org/10.31288/oftalmolzh20203915 Background: The technique of visual evoked potentials (VEPs) is the only non-invasive method that can provide important diagnostic information regarding the functional integrity of the visual system. Purpose: To assess the electrical visual system activity in full-term children versus those with retinopathy of prematurity (ROP) using flash and pattern VEPs. Material and Methods: Sixty-four 5 to 8-year-old children (120 eyes) were examined, and their medical records were retrospectively reviewed and divided into three groups: Group 1 (full-term controls), 11 children (22 eyes); Group 2 (regressive ROP), 26 children (50 eyes), and Group 3 (treated with laser for type 1 pre-threshold ROP or aggressive posterior ROP), 27 children (48 eyes). Flash and pattern VEPs were recorded in all subjects. Results: There was a significant difference (p < 0.05) in P1 latency between Group 1 and Group 3, but not between Group 1 and Group 2 or between Group 2 and Group 3. In addition, there was a significant difference (p < 0.005) in P1 amplitude between Group 1 and Group 2, and between Group 2 and Group 3, but not between Group 2 and Group 3. Moreover, there was a significant difference (p < 0.05) in P100 latency for 1-degree and 0.15-degree check sizes between Group 1 and Group 3, but not between Group 1 and Group 2 or between Group 2 and Group 3. There was a significant difference (p < 0.05) in P100 amplitude for 1-degree check size between Group 1 and Group 2, and between Group 1 and Group 3, but not between Group 2 and Group 3. Finally, there was a significant difference (p < 0.05) in P100 amplitude for 0.15-degree check size between Group 1 and Group 2, and between Group 1 and Group 3, but not between Group 2 and Group 3. Conclusion: First, latencies and amplitudes of the P100 component of pattern VEP, and of the P1 component of the fVEP were determined for 5 to 8-year-old full-term children with the use of RETIscan (Roland Consult). Second, VEP characteristics (latencies and amplitudes of the P100 component of pattern VEP, and of the P1 component of the fVEP) for 5 to 8-year-old children who underwent timely laser photocoagulation (LPC) for ROP were found to be within the age-related norm. Finally, there was no significant difference in VEP characteristics between 5 to 8-year-old children with spontaneously regressed ROP and their peers who underwent LPC for severe ROP, which points to timeliness and efficacy of the performed LPC. Keywords: retinopathy of prematurity, visual evoked potentials
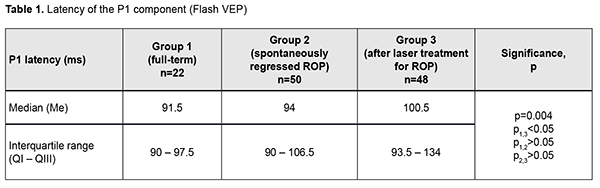
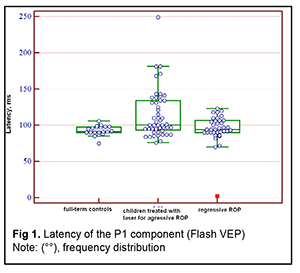
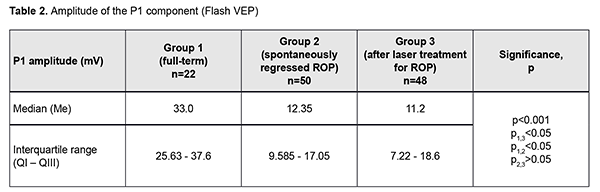
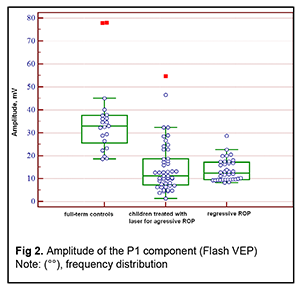
Introduction An increasing rate of successfully managed very low birth weight premature infants is a hallmark of modern medicine. In order to catch up in development with their normal peers, these children need rehabilitation targeted to the organs and systems that are not completely formed by the time of birth. The visual system is one of the most important ones affected by a serious complication of prematurity, specifically, retinopathy. Neurulation begins at day 18 of gestation. The eyes are formed within dozens of hours. The process of neurulation converts the neural plate into the neural tube and takes 10-13 days [9]. The neural tube failure to close may result in the birth of a cyclopic infant. Normally, at day 20-21 post-fertilization, a single eye field forms in the middle of the anterior neural plate, which separates into two optic vesicles. During this period, retinal ganglion cells develop from the vesicles, and the optic nerve develops from axons of retinal ganglion cells. From the eye, the optic nerve carries visual information to the lateral geniculate body, which is the primary visual centre in humans. And from there the nerve fibers that make up the optic radiation carry the visual signal to the primary visual cortex (specifically, occipital cortical areas 17, 18, and 19). The retinal ganglion cell axons form the retinal nerve fiber layer. The number of ganglion cells increases by the beginning of the 3rd month of embryo life, and the number of retinal ganglion cell fibers per eye is as high as 80,000 at 11-12 weeks’ gestational age, and about 170,000 at 4 months. However, the number decreases to 140,000 fibers per eye at 16 weeks’ gestational age, and about 117,000 fibers per eye at 6 months [9]. However, because unfavorable fetus development causes preterm delivery, it seems fair to suppose that the number of visual system neurons is rather low initially, and, subsequently, as much as 40% of visual fibers are lost due to physiological autolysis. Consequently, a rather alarming condition, retinopathy, develops in a premature infant, which frequently results in retinal detachment and blindness [9]. The electrophysiological technique of visual evoked potential (VEP) has been used since the mid-1960s to assess visual system function and maturity, and for diagnosis of injuries to the retinocortical pathway. Although the technique has been used for dozens of years, it is being actively studied because it is still the only non-invasive method capable of determining the functional integrity of the visual system. For clinical purposes, flash VEP and checkerboard pattern reversal VEP are most commonly used. Flash VEP is more variable in appearance than the pattern reversal VEP, but there is a group of patients for whom the former is sometimes the only appropriate diagnostic technique. This group includes (1) infants, (2) the children who cannot concentrate their attention and/or fix their eyes on anything for a long enough time, and (3) children with low visual acuity [8]. VEP reflects the total response of large populations of cortical neurons to the afferent stimulus-induced synchronous pulse train with a flash or reversal pattern stimulus acting as an afferent stimulus to gaze. The whole retinal surface responds to flash, and excitation passes through all optic nerve fibers. In checkerboard pattern reversal VEP, a structured stimulus excites the cones of the central and paracentral retina, and the information from area 17 of the visual cortex is subsequently processed [9]. VEP represents a polyphasic negative-and-positive oscillation. VEP components can be divided into the early (occurring within 100 ms after stimulus onset) and the late (100 ms to 300 ms after stimulus onset) components. VEP consists of specific and non-specific responses due to the existence of the two different afferent systems. In the first afferent system, pulses are transmitted via the retino-geniculo-striatal pathway. The P100 component of the VEP has the largest amplitude and a latency of about 100 ms and is thought to reflect the function of the first system. The activity of the second afferent system is associated with the activity of mesencephalic structures and non-specific thalamic nuclei which induces the onset of later VEP components [9]. The International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocols has been defined for a single recording channel with a midline occipital active electrode. The purpose of this study was to assess the electrical visual system activity in full-term children versus those with retinopathy of prematurity (ROP) using flash and pattern VEPs. Material and Methods The medical records of 64 children (120 eyes) who were under observation at the polyclinic of the Filatov Institute were retrospectively reviewed. Children were divided into three groups. Group 1 involved 11 full-term controls (22 eyes; mean age, 6.3 years; mean visual acuity, 1.2). Group 2 involved 26 children (50 eyes; mean age, 6.8 years; mean beast-corrected visual acuity (BCVA), 1.0) with regressive ROP. Group 3 involved 27 children (48 eyes; mean age, 5.9 years; mean beast-corrected visual acuity (BCVA), 0.9) treated with laser for type 1 pre-threshold ROP or aggressive posterior ROP (APROP). ROP was diagnosed as per the ISCEV standard protocols. Patients of Group 3 underwent confluent laser (Purepoint Laser; Alcon, Fort Worth, TX) photocoagulation. The laser parameters were as follows: 532 nm wavelength, 120-220 mW exposure power, 100-150 ms exposure time, maximum spot size, and 100-ms pulse-to-pulse interval. Laser energy was delivered in pulse mode. The total number of laser spots per eye was as much as 4,000 to 5,000 depending on the severity and extension of pathologic changes in the fundus. This VEP study was performed using RETIscan (Roland Consult, Wiesbaden, Germany). Reference and ground electrodes were placed on the right and left earlobes, respectively, and the active electrode was placed on the occipital scalp, 2-3 cm above the inion. Monocular stimulation was used. A “miniGanzfeld” dome was used to deliver flash light stimuli of proper intensity at a frequency of 8 Hz. Pattern stimuli were checkerboards consisting of black and white squares that were rapidly reversed. The stimuli were generated on a screen, and special software was used to quantify and analyze the VEP. EZR v.1.35 (R statistical software version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses [2, 6]. Quantitative data were presented as medians and interquartile ranges (IQR). Normality of distribution was assessed using the Shapiro-Wilk test. Because the distribution was not normal, the Kruskal–Wallis test was used for comparing data from three different groups, followed by post hoc comparisons using the Dunn test [6]. The level of significance p ? 0.05 was assumed. Results The three groups were compared on amplitude and latency of the P1 component of the flash visual evoked response. There was a significant difference (p = 0.004) in P1 latency between Group 1 (full-term children) and Group 3 (ROP after laser treatment), but not between Group 1 and Group 2 (spontaneously regressed ROP) or between Group 2 and Group 3 (Table 1 and Fig. 1). In addition, there was a significant difference (p < 0.005) in P1 amplitude between Group 1 and Group 2, and between Group 2 and Group 3, but not between Group 2 and Group 3 (Table 2 and Fig. 2).
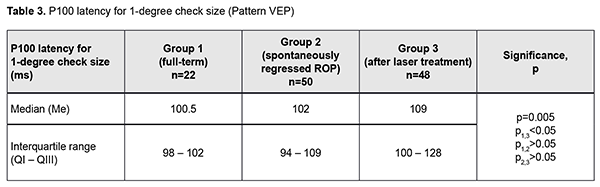
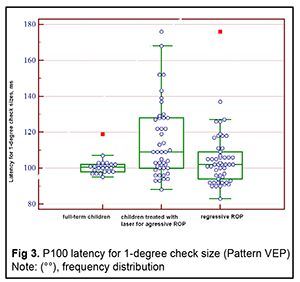
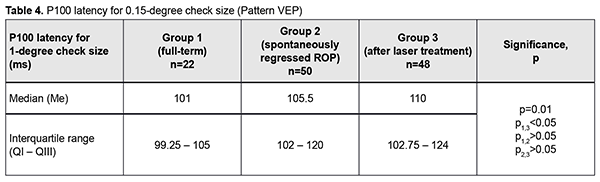
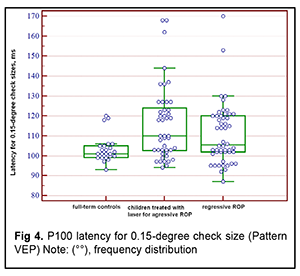
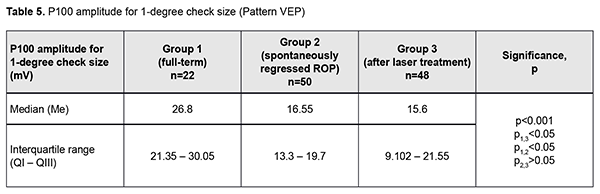
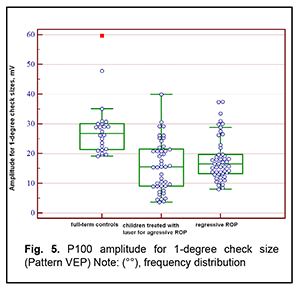
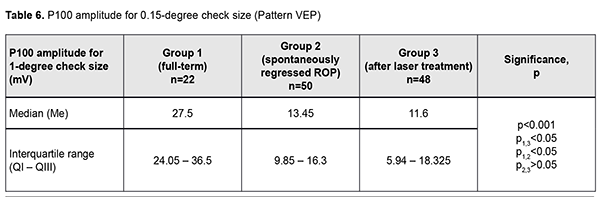
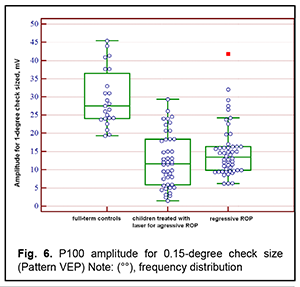
Next, the three groups were compared on amplitude and latency of the P100 component of the pattern visual evoked response. There was a significant difference (p < 0.05) in P100 latency for 1-degree check size between Group 1 and Group 3, but not between Group 1 and Group 2 or between Group 2 and Group 3 (Tables 3 and 4 and Figs 3 and 4). In addition, there was a significant difference (p < 0.005) in P100 latency for 0.15-degree check size between Group 1 and Group 3, but not between Group 1 and Group 2 or between Group 2 and Group 3 (Table 4 and Fig 4). Moreover, there was a significant difference (p < 0.05) in P100 amplitude for 1-degree check size between Group 1 and Group 2, and between Group 1 and Group 3, but not between Group 2 and Group 3 (Table 5 and Fig 5). Finally, there was a significant difference (p < 0.05) in P100 amplitude for 0.15-degree check size between Group 1 and Group 2, and between Group 1 and Group 3, but not between Group 2 and Group 3 (Table 6 and Fig. 6).
Discussion VEP is a non-invasive tool for measuring the electrical activity of the brain. Numerous researchers have studied VEP parameters in preterm infants [10] in order to obtain interpretable signals and demonstrate the sequence of the development of visual brain pathways [1, 10]. Flash VEP is sometimes the only appropriate technique for diagnosing the state of the visual system in early age children. In flash VEP, the response from the complete field of vision and bioelectrical activity of the whole visual system are determined. The technique, however, does not assess visual system domains separately. The presence of a central vision disorder cannot be excluded even if there are no changes in the visual pathway. Koshelev et al [8] reported on the use of flash VEP for the assessment of visual system function in preterm children in 2014. They noted that the technique (a) identifies the presence of vision in the early age, when assessing visual acuity using an Orlova chart is impossible; (b) allows assessing how well the visual functions are preserved after peripheral retinal laser photocoagulation (LPC); (c) allows assessing the velocity of visual signal conduction and the efficacy of visual signal processing; and (d) allows comparing the right and left monocular channels for activity and assessing binocular integration of these channels [8]. In the current study, we included three different groups (full-term infants, children with spontaneously regressed ROP, and children with aggressive posterior ROP), and made a more detailed comparative group analysis not only for fVEP P1 amplitude and latency, but also for pattern VEP (P100). In addition, as opposed to the study by Koshelev et al [8], where the subjects were children aged 3 years or younger, children included in this study were aged 5 to 8 years, and the information obtained on visual system function was more accurate. Our statistical analysis found a significant difference in amplitude and latency between groups 1 and 2 and between groups 1 and 3. In addition, there was no significant difference in any characteristic between children with spontaneously regressed ROP and children treated with laser for aggressive posterior ROP. Moreover, P100 latency was shorter in children with spontaneously regressed ROP than in full-term children. These findings are close to those of Magoon et al [7] who noted that maturation of cerebral cortex structures and formation of synaptic connections begin at 37 weeks of gestation, right after the visual pathways have been myelinated. Madan et al [3] demonstrated an improvement in VEP P100 amplitude during the period of formation of the lateral cortical connections. The short P100 latency in the preterm children has been attributed to fast maturation of the cerebral cortex and delayed myelination of visual pathways [1]. Early maturation of fVEP N1 in the preterm children has been noted previously and was attributed to early visual cortex development. In agreement with those findings, we noted that the fVEP N1 latency in the preterm children (30-45 ms) was shorter than in full-term children (45-50 ms) [5]. In addition, in the current study, the fVEP P1 latency in 5 to 8-year-old full-term children and children with spontaneously regressed ROP was within 90-94 seconds. This indicated the cerebral white matter maturation pattern for full-term children was similar to that for preterm children [5]. However, the fVEP P1 latency in children treated with laser for aggressive ROP was as long as 134 ms, which was attributed to immature structures and delayed neural conduction along visual pathways. Low fVEP P1 amplitude in children of groups 2 and 3 indicates immature visual pathways in the total preterm group [4]. Kogoleva [7] used pattern VEP topographic mapping to find long P100 latency in 3 of 18 eyes with stage 1 ROP, 9 of 41 eyes with stage 2 or 3 ROP, and 10 of 53 eyes with stage 4 or 5 ROP. Therefore, there was no strong correlation between P100 latency and stage of ROP. Faizullinа and colleagues [10] found abnormal visual pathway function in 72.9% of one-year-old children with cicatricial ROP, the most common abnormalities being low functional activity of visual structures (28.7%) and abnormal optic nerve function (20.1%). In addition, abnormal optic nerve function was 3.6 times more common in infants after panretinal LPC than in those after focal LPC (47.3% vs 13.1%). This is in agreement with our findings for children who underwent confluent LPC for aggressive ROP. The literature and our findings suggest that P1 amplitude and P100 amplitude decrease with increased disease severity. We, however, found no significant correlation between P1 latency or P100 latency and severity of ROP. Our findings of long latency and low amplitude of Р100 and Р1 point to the likelihood of pathway abnormalities and/or partial optic nerve atrophy in preterm children with ROP. However, the activity of primary visual centers of the occipital lobes was found to be still prevailing, which was confirmed by the absence of severe central retinal changes. In severe ROP, long Р100 latencies and low Р100 amplitudes, long Р1 latencies and low Р1 amplitudes, along with changes in morphometric changes in the optic disc (increased maximum elevation and depression of the contour line) indicate optic disc traction and dysfunction of the papillomacular bundle [4]. In addition, long Р100 latencies and low Р100 amplitudes and long Р1 latencies and low Р1 amplitudes in children with ROP indicate that there is a combination of visual abnormalities due to not only the severity of ROP, but also due to concomitant disorders of the visual pathways and occipital lobes of the cerebral cortex. Employment of different types of VEP improves the potential for assessment of the state and site of damage to the visual pathway, prediction of changes in visual acuity, selection of medical rehabilitation strategy. Conclusion First, latencies and amplitudes of the P100 component of pattern VEP, and of the P1 component of the fVEP were determined for 5 to 8-year-old full-term children with the use of RETIscan (Roland Consult). Second, VEP characteristics (latencies and amplitudes of the P100 component of pattern VEP, and of the P1 component of the fVEP) for 5 to 8-year-old children who underwent timely LPC for ROP were found to be within the age-related norm. Finally, there was no significant difference in VEP characteristics between 5 to 8-year-old children with spontaneously regressed ROP and their peers who underwent LPC for severe ROP, which points to timeliness and efficacy of the performed LPC. References 1.Madan A, Jan JE, Good WV. Visual development in preterm infants. Dev Med Child Neurol. 2005 Apr;47(4):276-80. 2.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013 Mar;48(3):452-8. 3.Magoon EH, Robb RM. Development of myelin in human optic nerve and tract. A light and electron microscopic study. Arch Ophthalmol. 1981 Apr;99(4):655-9. 4.Park KA, Oh SY. Retinal nerve fiber layer thickness in prematurity is correlated with stage of retinopathy of prematurity. Eye (Lond). 2015 Dec; 29(12): 1594–1602. 5. Tsueineshi S, Casaer P. Effects of preterm extrauterine visuak expirience on the development of the human visual system: a flash VEP study. Dev Med Child Neurol.2000 Oct;42(10):663-8. 6.Gur’ianov VG, Liakh II, Parii VD, Korotkyi OV, Chalyi OV, Chalyi KO, Tsekhmister IV. [Analysis of the results of medical studies using EZR (R–statistics) software: Biostatistics training manual]. Kyiv: Vistka; 2018. Ukrainian. 7.Kogoleva LV. [System for preventing and predicting vision impairment in retinopathy of prematurity]. [Dissertation for fulfillment of a Dr Sc (Med) degree]. Moscow: Helmholtz Research Institute of Eye Disease; 2016. Russian. 8.Koshelev DI, Galautdinov MF, Vakhmyanina AA. [The use of flash visual evoked potentials in the assessment of visual system functions]. Vesnik OGU. 2014; 12(173):181-7. Russian. 9.Safonov DL. [Diagnostic and prognostic value of evoked potentials in pediatric epileptics]. [Thesis for fulfillment of a Cand Sc (Med) degree]. Moscow: Russian Medical Academy of Post-graduate Education; 2011. Russian. 10.Faizullinа AS, Zainutdinova GKh, Ryskulova EK. [Study of the visual pathway function in cicatricial retinopathy of prematurity]. Tochka zreniia. Vostok-Zapad. 2016;3:141-6. Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|