J.ophthalmol.(Ukraine).2020;3:53-60.
|
http://doi.org/10.31288/oftalmolzh202035360 Received: 26 February 2020; Published on-line: 24 June 2020 Dynamics of depositing and diffusion of drugs (chlorhexidine, 5-fluorouracil and doxorubicin) in hydrogel implants with different hydrogel crosslinking densities Iu.M. Samchenko1, A.P. Maletskiy2, N.M. Bigun3, G.A. Dolynskyy1, L.O. Kernosenko1, N.O. Pasmurtseva1; T.P. Poltoratska1; I.Ie. Mamyshev1 1 Ovcharenko Institute of Biocolloid Chemistry, NAS of Ukraine; Kyiv (Ukraine) 2 SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Odesa (Ukraine) 3 Lviv Regional Clinical Hospital; Lviv (Ukraine) E-mail: maletskiy@filatov.com.ua TO CITE THIS ARTICLE: Samchenko Iu.M., Maletskiy A.P., Bigun N.M., Dolynskyy G.A., Kernosenko L.O., Pasmurtseva N.O., Poltoratska T.P., Mamyshev I.Ie. Dynamics of depositing and diffusion of drugs (chlorhexidine, 5-fluorouracil and doxorubicin) in hydrogel implants with different hydrogel crosslinking densities. J.ophthalmol.(Ukraine).2020;3:53-60. http://doi.org/10.31288/oftalmolzh202035360 Background: Due to increasing prevalence of ocular trauma, more and more patients need to have their affected orbit, orbital adnexa, and periorbital area restored, which results in an increasing demand for implant materials. It is important for these materials to contain antimicrobial and antitumor drugs in order to prevent inflammation and recurrent inflammation around the implant before and after tumor removal. Purpose: To study the dynamics of depositing and diffusion of drugs (chlorhexidine, 5-fluorouracil and doxorubicin) in hydrogel implants with different hydrogel crosslinking densities. Material and Methods: Pharmaceuticals: 2.0% chlorhexidine digluconate; 5-fluorouracil (Ebewe Pharma, Unterach, Austria) infusion solution concentrate, 50 mg/ml; and doxorubicin (Ebewe), 2 mg/ml. Results: The time required for the drug concentration to reach the minimum therapeutic level was as small as several minutes for the low-density cross-linked hydrogel, whereas the diffusion lag time for the high-density cross-linked hydrogel was as large as several hours. The latter hydrogel was found to have a higher capacity for deposition of chlorhexidine digluconate and 5-fluorouracil, and is reasonable to be utilized in implants with a prolonged antibacterial effect, whereas the former hydrogel is reasonable to be utilized for a fast-release bolus antiseptic delivery. In in vitro experiments, the low-density cross-linked hydrogel hydrogel provided a 3-4-fold greater drug concentration in the environment, and allowed for a smoother and more prolonged drug release profile compared to the high-density cross-linked hydrogel hydrogel. Conclusion: Such a capacity for prolonged drug release will promote application of hybrid hydrogel implants for depositing anti-cancer drugs and maintaining effective concentrations of the latter at pathology focus. Keywords: hybrid hydrogel, endoprosthesis, drug deposition and diffusion, degree of hydrogel crosslinking, reconstructive surgery
Introduction Craniofacial injuries represent 29% of all trauma cases [1, 2], and are primarily caused by anthropogenic and criminal-related ocular and orbital trauma. In this connection, more and more patients need to have their affected orbit, orbital adnexa, and periorbital area restored [3, 4], in particular, after surgery for eye cancer. Surgeons are not always satisfied with using biological tissue as a ductile material, and legal requirements for harvesting of donor material have been made increasingly stronger. The development of synthetic polymer materials for restoration the structures that have suffered anatomical and/or functional damage is therefore of primary importance. Previously, our experimental in vivo study [5] has demonstrated high biocompatibility and no resorption for our highly porous polyvinyl formal composite material, as well as ingrowth of adjacent tissue into implanted constructs. These findings showed that this was a promising material requiring further research aimed at an improvement in its performance. It is important for implanted materials to contain antimicrobial and antitumor drugs in order to prevent inflammation and recurrent inflammation around the implant after tumor removal. The hydrogels under study are hydrophilic cross-linked polymers able to absorb large amounts of water and they are soft, flexible, mechanically robust and biocompatible [6]. Because their hydrophilic, soft, porous and elastic physical characteristics are more advantageous than those of other synthetic biomaterials, and are especially suitable for human tissue, they have been used for decades for manufacturing soft contact lenses [7], delivery systems for targeted delivery and prolonged release of drugs [8-11], biosensors [12], burn dressings and hemorrhage dressings [13], tissue engineering and plastic surgery materials [14], etc. Given the potential of the proposed hydrogels for use in manufacturing the implants capable of depositing antimicrobial and antitumor drugs, it seemed reasonable to assess diffusion characteristics of hydrogels with different porosities and with chlorhexidine, 5-fluorouracil and doxorubicin immobilized on their surface. Material and Methods Studies were conducted at the Department for Functional Hydrogels, Ovcharenko Institute of Biocoloid Chemistry, NAMS of Ukraine, with the use of the following substances and materials: Hydrogel synthesis reagents: linear polyvinyl alcohol (PVA; 98 %; AppliChem GmbH, Darmstadt, Germany; 72 kDa), formaldehyde (37%; LAB-SCAN); concentrated sulfuric acid; Triton X-100 (AppliChem GmbH); acrylic acid (99%; Synbias); ammonium persulfate (98%;Thermo Fisher); N, N'-Methylene bisacrylamide (Merck) were used without additional purification. The hydrogel implant is shown in Fig. 1.
Pharmaceuticals: OsteohexTM (2.0% chlorhexidine digluconate); 5-fluorouracil (Ebewe Pharma, Unterach, Austria) infusion solution concentrate, 50 mg/ml; and doxorubicin (Ebewe), 2 mg/ml. The details of polyvinyl formal synthesis have been published previously [15]. Hydrogels with different densities were obtained by varying PVA content in the blend. The PVA content in low-density cross-linked hydrogel and high-density cross-linked hydrogel was 7.1wt% and 8.0 wt%, respectively. In order to fabricate a hybride hydrogel from previously synthesized polyvinyl formal and acrylic acid, 0.6 g of the high-density cross-linked hydrogel was put in a 10-mL syringe, and impregnated with fluid composed of 0.6 ml of acrylic acid, 0.2 ml of N, N'-Methylene bisacrylamide, and 5.25 ml of ammonium persulfate (40% w/v) (Table 1). After impregnation, 4.5 ml of fluid was squeezed, and the obtained composite was put into the drying cabinet for 60 minutes at 40°C.
Fourier-transform infrared spectroscopy (FTIR) spectra were collected with a Perkin-Elmer spectrum BX FT-IR system (Perkin-Elmer Corporation, Beaconsfield, Bucks, UK). Spectra were registered from 4000 to 550 cm?1 at 2cm?1 resolution using total internal reflection spectroscopy. Eight scans were averaged per spectral replicate to avoid accidental artifacts. Porosity of samples was determined gravimetrically using Eq. 1.
where P is overall porosity [%]; V is dry sample volume; and ? is density of the used material [16]. An optical microscope SIGETA MB 140 LED Mono (Ukraine) was used to determine pore size in transmitted, reflected and mixed light. Microscopic images and statistics were analyzed using Toup View 3.5 sotware. Photographs taken with the JSM-6060 LA (JEOL, Japan) scanning electron microscope were analyzed to obtain detailed information on the structure of the polymer pore space. Polymer samples were lyophilized in a freeze dryer (UZV-10; Kharkiv, Ukraine), affixed to standard holders using double-sided conductive tape, and coated with a thin layer (25 nm) of Au/Pd alloy using a Gatan 682 Precision Etching and Coating System (PECS) (Gatan, USA). Kinetics of swelling of hydrogel samples was studied at 25 °С in distilled water and physiologic saline (0.9% sodium chloride). The degree of swelling (Q) for samples weighting 23.8–27.0 mg was determined gravimetrically using the formula as follows:
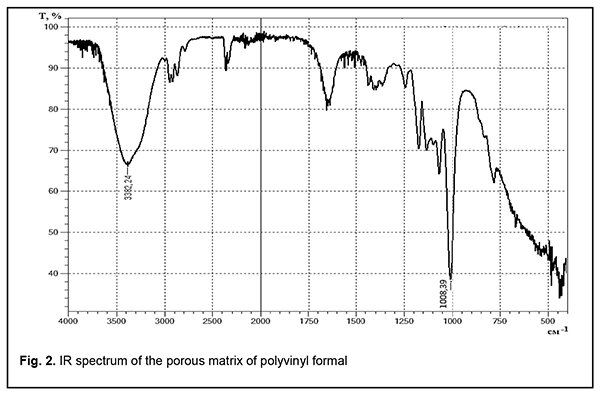
where Qt is the degree of swelling, mt is the wet mass of the gel at time t and md is the dry mass of the gel [17]. Drug diffusion in hydrogels was studied as follows. Dry hydrogel samples shaped as cylinders of 12 mm in diameter and 5-8 mm in height (the height varied from 5 to 8 mm depending on hydrogel content) were immersed in aqueous solutions of various pharmaceuticals (2.0% chlorhexidine digluconate in water for injections; 0.5% 5-fluorouracil in water for injections; 0.02% doxorubicin in 0.9% sodium chloride) at 25 °С for 18 hours. The mass of the deposited substance was calculated taking in account changes in hydrogel concentration. Excess fluid was squeezed from the swollen sample, and thereafter, the sample was weighted and put in a vial containing 20 ml of physiological saline. Drug diffusion was assessed by UV spectroscopy using the Spectrophotometer / Fluorometer System DS-11 FX+ (DeNovix, USA) at 25°С at spaced time points within a day and with intermittent agitation. Reactant concentrations were determined based on the absorption peaks of chlorhexidine digluconate at 255 nm, 5-fluorouracil at 268 nm, and doxorubicin at 480 nm. Results and Discussion Infrared (IR) spectroscopy Functional groups of porous matrices of polyvinyl formal were characterized based on the obtained IR spectra (Fig. 2). Absorption bands in the range of 3362 – 3382 cm-1 with wide and intensive peaks may be attributed to stretching vibrations of hydroxyl groups. Widening of these absorption bands is explained by hydrogen bonds of OH- groups. Absorption bands at 1007 cm-1 in the spectrum of porous matrix of polyvinyl formal may be attributed to stretching vibrations of the hydroxyl group C OH from primary alcohols.
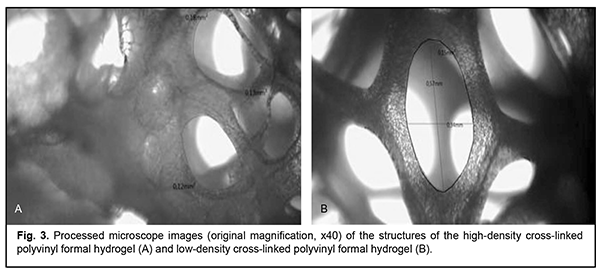
Porous structure of hydrogels Porosities of the high-density cross-linked hydrogel and low-density cross-linked hydrogel were 91.8%, and 95.0%, respectively, according to calculations based on formula (1). The calculated porosity of (poly) acrylic acid hydrogel composite was 85.9%. From optical microscopic evidence and electron microscopy evidence, the obtained hydrogels had a heterogeneous multilevel porous structure. That is, highest-level pores resulted also from coalescence of pore structures with a size that was two orders of magnitude lower. According to our calculations, the highest-level pores had a size of 120-180 ?m for the high-density cross-linked hydrogel and 460-670 ?m for the low-density cross-linked hydrogel (Fig. 3B).
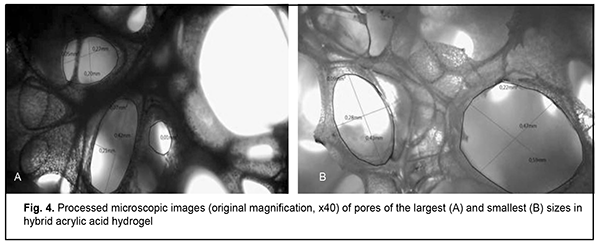
The pore size in hybrid acrylic acid hydrogel composite varied from 200 ?m (Fig. 4A) to 590 ?m (Fig. 4B).
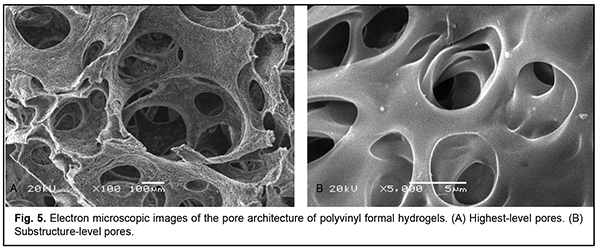
Lowest-level pores form the substructure of walls of large pores (Fig. 5A), and had a size of 3-5 ?m irrespective of hydrogel density (Fig. 5B).
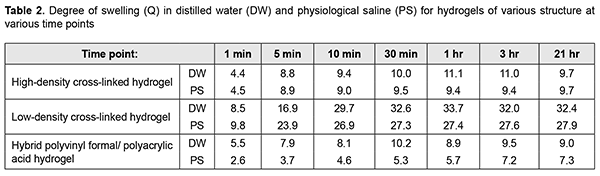
Porosity and pore size of the implant material play an important role in tissue regeneration, and are widely studies and discussed in the literature. The porous structure of the matrix is essential for tissue regeneration, because it exerts effects on cell adhesion, migration and proliferation, and diffusion of nutrients, oxygen and metabolites. It has been found that high-size pores facilitate nutrient delivery and metabolic product removal, whereas low-size pores allow for increased surface area for cell adhesion [18-20]. It is noteworthy that the effect of pore architecture on cell behavior also depends on cell nature. In an in vitro study [21], the scaffold section with 380-405 microm pore size showed better cell growth for chondrocytes and osteoblasts, while the scaffold section with 186-200 microm pore size was better for fibroblasts growth. These preferences may be explained by the following: although larger pores facilitate nutrient and oxygen diffusion, fibroblasts tend to adhere to scaffold sections with smaller pores due to increased scaffold specific surface area [22]. Although in vitro studies demonstrated that the increased porosity was accompanied by increased cell migration and infiltration [23, 24], it may cause protein wash-out in vivo [22]. Pore interconnectivity is another important characteristic feature of the scaffold, and influences not only movement within the scaffold whether it be diffusion of soluble factors, oxygen, and nutrients or cell migration, but also growth factor-mediated vascularization [25]. Consequently, it can be stated that the hydrogels that we have developed can accumulate and transport various metabolites and pharmaceuticals via the micropore system, and serve as a matrix for adherence and migration of various cell types that are involved in the course of regeneration processes in biological tissues. Hydrogel swelling kinetics From the analysis of swelling kinetics of hydrogels with various structures (Table 2), it can be concluded that during swelling, equilibrium was achieved within 30 minutes for all hydrogel samples immersed in water. Although the degree of swelling of the samples immersed in physiological saline was somewhat (by approximately 16%) lower compared to the samples immersed in water for injections, this exerted almost no effect on a high swelling rate. Swelling equilibrium was, however, achieved within 3 hours for (poly) acrylic acid hydroxyl composite samples immersed in physiological saline. This pattern is characteristic of all hydrogels and explained by low ionic osmotic pressure with an increase in ionic power of the solution resulting in hydrogel swelling [26]. These findings were used to determine the time needed for hydrogel saturation with drugs.
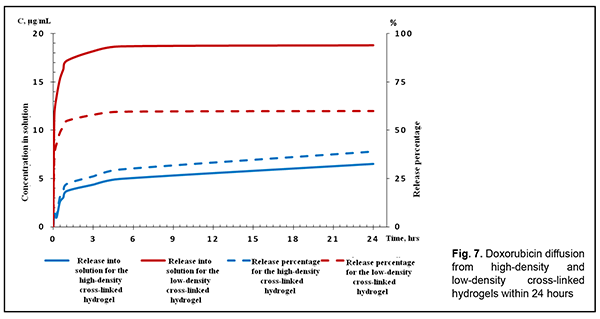
Drug diffusion in high-density and low-density cross-linked hydrogels Fig. 4 presents the diffusion kinetics of chlorhexidine and 5-fluorouracil. Minimal inhibitory concentrations (MIC) of chlorhexidine for pathogenic bacteria [13] were achieved in less than 6 hours (Fig. 4), which was evidence of a reliable antimicrobial effect immediately after composite implantation. It is noteworthy that the time required to reach concentration values close to a minimum therapeutic level was as small as several minutes for the low-density cross-linked hydrogel, whereas the diffusion lag time for the high-density cross-linked hydrogel was as large as several hours, with a significantly (20-25%) lower amount of released drug at the time when the equilibrium was reached. Sorption studies demonstrated that the latter hydrogel has a higher capacity for drug deposition, and is reasonable to be utilized in implants with a prolonged antibacterial effect, whereas the former hydrogel is reasonable to be utilized for a fast-release bolus antiseptic delivery. Now let us consider diffusion properties of hydrogel implants loaded with an anti-cancer drug. Because 5-fluorouracil (5-FU) is released from the plasma as early as 30 minutes after intravenous infusion, clinicians have to administer high doses of this agent, which causes toxic side effects [27, 28]. The same is true with regard to doxorubicin. In our sorption studies, the developed hydrogels (especially the high-density cross-linked hydrogel) demonstrated a high capacity for accumulation of 5-FU. Both hydrogels provided prolonged drug release (with drug diffusion time > 6 hours), but the high-density cross-linked hydrogel provided a somewhat (20-30%) higher equilibrium drug concentration (Fig. 6) compared to the low-density cross-linked hydrogel. Given the demand for means of low-dose controlled release of 5-FU [29] in combination chemotherapy [30] or metronomic [31] chemotherapy, the composite hydrogels based on polyvinyl formal seems promising for reconstructive surgery in cancer patients and ophthalmic patients [32] for preventing tissue scarring after glaucoma filtration surgery. Now let us consider sorption and diffusion of the cytostatic drug doxorubicin. The low-density cross-linked hydrogel sorbs twice as much doxorubicin as the high-density cross-linked hydrogel, possibly due to the absence of steric obstacles to penetration of the porous hydrogel structure by a large drug molecule (molecular weight, 544 g/mol). In in vitro experiments, the former hydrogel provided a 3-4-fold greater drug concentration in the environment compared to the latter hydrogel (Fig. 7). The latter hydrogel, however, allows for a smoother and more prolonged drug release profile.
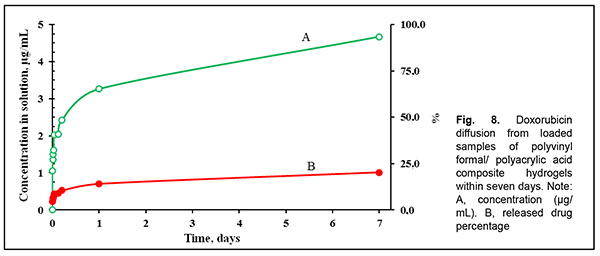
Hybrid polyvinyl formal/ polyacrylic acid composite hydrogels showed an order of magnitude higher doxorubicin deposition capacity compared to the polyvinyl formal component. This effect probably results from the development of ionic bonds between the active –СООН groups from the hybrid hydrogel and amine groups from doxorubicin. It is the slow hydrolysis of these ionic bonds that explains the prolonged (several days) release of doxorubicin from hybrid hydrogels (Fig. 8).
Such a capacity for prolonged drug release will promote application of hybrid hydrogel implants for depositing anti-cancer drugs and maintaining effective concentrations of the latter at pathology focus. Conclusion First, the time required for the drug concentration to reach the minimum therapeutic level was as small as several minutes for the low-density cross-linked hydrogel, whereas the diffusion lag time for the high-density cross-linked hydrogel was as large as several hours, with a significantly (20-25%) lower amount of released drug at the time when the equilibrium was reached. Second, the latter hydrogel was found to have a higher capacity for drug (doxorubicin or 5-FU) deposition, and therefore is reasonable to be utilized in implants with a prolonged antibacterial effect, whereas the former hydrogel is reasonable to be utilized for a fast-release bolus antiseptic delivery. Finally, the low-density cross-linked hydrogel sorbs twice as much doxorubicin as the high-density cross-linked hydrogel, possibly due to the absence of steric obstacles to penetration of the porous hydrogel structure by a large drug molecule (molecular weight, 544 g/mol). In in vitro experiments, the former hydrogel provided a 3-4-fold greater drug concentration in the environment, and allowed for a smoother and more prolonged drug release profile compared to the latter hydrogel. References 1.O.V. Grusha, Ia.O. Grusha. [Five hundred of orbital plastic repairs: analysis of complications]. Vestn Oftalmol. Jan-Feb 2006;122(1):22-4. Russian. 2.Gundorova RA, Neroev VV, Kashnikov VV, editors. [Ocular injuries]. Moscow: GEOTAR-Media; 2009. Russian. 3.Krasnovid TA. [Ocular trauma under present conditions. Providing urgent care in Ukraine]. Proceedings of the Conference of Ophthalmologists of Chernihiv, Kyiv, and other regions. Chernihiv; 2013. pp. 40-4. Russian. 4.Tselomudryi OI, Venger GE, Rizvaniuk AV, Pogorelyi DN, Putienko VA. [Current system of stage-by-stage treatment for combat-related eye injuries in the area of ATO]. In: [Proceedings of the Conference commemorating the 80th anniversary of the Filatov Institute and 14th Congress of the Black Sea Ophthalmological Society]. Odesa, 2016. Russian. 5.Maletskiy AP, Samchenko Iu.M, Vit VV, Bigun NM, Kernosenko LO. [Response of orbital and auricular soft tissues to the developed hydrogel implant in rabbits]. Arkhiv oftalmologii Ukrainy. 2018;6(2):20-7. Ukrainian. 6.Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels. 2017;3(1):6. 7.Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960; 185: 117–8. 8.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49: 1993–2007. 9.Jeong CG, Zhang H, Hollister SJ. Three-dimensional poly (1,8-octanediol–co-citrate) scaffold pore shape and permeability effects on sub-cutaneous in vivo chondrogenesis using primary chondrocytes. Acta Biomater. 2011 Feb;7(2):505-14. 10.Murray RZ, West ZE, Cowin AJ, Farrugia BL. Development and use of biomaterials as wound healing therapies. Burns Trauma. 2019;7:2. 11.Overstreet DJ, McLemore RY, Doan BD, Farag A, Vernon BL. Temperature-responsive graft copolymer hydrogels for controlled swelling and drug delivery. Soft Matter. 2013; 11: 294–304. Crossref 12.Yu X, Jiao Y, Chai Q. Applications of gold nanoparticles in biosensors. Nano LIFE. 2016; 6: 1642001. 13.Sugak OA, Panasenko OI, Knysh YG, Kamyshny OM. [Antimicrobial and antifungal activity of derivatives of 3- (alkylthio) -4-R-5- (thiophen-2-ylmethyl) - 4H-1,2,4-triazoles]. Aktualni pytannia farmacevtychnoi i medychnoi nauky ta praktyky. 2015;3(19):67–70. Ukrainian. 14.Shapiro JM, Oyen ML. Hydrogel composite materials for tissue engineering scaffolds. JOM. 2013; 65: 505-16. 15.Kryklia SO, Samchenko Iu.M, Konovalova VV, Poltoratska TP, Pasmurtseva NO, Ulberg ZR. [Hybrid Ph- and thermosensitive hydrogels based on polyvinylalcohol and acrylic monomers]. Magisterium. 2016;63:20-8. Ukrainian. 16.Br?nler R, Aibibu D, W?ltje M, Anthofer AM, Cherif C. In silico modeling of structural and porosity properties of additive manufactured implants for regenerative medicine. Mater Sci Eng C Mater Biol Appl. 2017; 76: 810–817. 17.Abureesh MA, Oladipo AA, Gazi M. Facile synthesis of glucose-sensitive chitosan-poly(vinyl alcohol) hydrogel: drug release optimization and swelling properties. Int J Biol Macromol. 2016 Sep;90:75-80. 18.Van Tienen TG, Heijkants RG, Buma P, de Groot JH, Pennings AJ, Veth RP. Tissue ingrowth and degradation of two biodegradable porous polymers with different porosities and pore sizes. Biomaterials. 2002 Apr;23(8):1731-8. 19.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001 Dec;7(6):679-89.Crossref PubMed 20.Zeltinger J, Sherwood JK, Graham DA, M?eller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001 Oct;7(5):557-72. 21.Oh SH, Park IK, Kim JM, Lee JH. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials. 2007 Mar;28(9):1664-71. 22.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010 Jan;31(3):461-6. 23.Bulysheva AA, Bowlin GL, Klingelhutz AJ, Yeudall WA. J. Low-temperature electrospun silk scaffold for in vitro mucosal modeling. Biomed Mater Res. Part A. 2012; 100: 757–67. 24.Rnjak-Kovacina J, Wise SG, Li Z, Maitz PK, Young CJ, Wang Y, Weiss AS. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials. 2011 Oct;32(28):6729-36. 25.Somo SI, Akar B, Bayrak ES, Larson JC, Appel AA, Mehdizadeh H, Cinar A, Brey EM. Pore interconnectivity influences growth factor mediated vascularization in sphere-templated hydrogels. Tissue Eng Part C Methods. 2015 Aug;21(8):773-85. 26.Khare A, Peppas NA. Swelling/deswelling of anionic copolymer gels. Biomaterials. 1995 May;16(7):559-67. 27.Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996 Feb 1;77(3):441-51. 28.Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther. 1990;48(3):381-95. 29.Wang LL, Huang S, Guo HH, Han YX, Zheng WS, Jiang JD. In situ delivery of thermosensitive gel-mediated 5-fluorouracil microemulsion for the treatment of colorectal cancer. Drug Des Devel Ther. 2016 Sep 8;10:2855-2867. 30.Miyake M, Anai S, Fujimoto K, Ohnishi S, Kuwada M, Nakai Y, et al. 5 fluorouracil enhances the antitumor effect of sorafenib and sunitinib in a xenograft model of human renal cell carcinoma. Oncol Let. 2012 Jun;3(6):1195-1202. 31.Ma Y, Wang Y, Xu Z, Wang Y, Fallon JK, Liu F. Extreme low dose of 5-fluorouracil reverses MDR in cancer by sensitizing cancer associated fibroblasts and down-regulating P-gp. PLoS One. 2017 Jun 29;12(6):e0180023. 32.Yang LQ, Lan YQ, Guo H, Cheng LZ, Fan JZ, Cai X, et al. Ophthalmic drug-loaded N,O-carboxymethyl chitosan hydrogels: synthesis, in vitro and in vivo evaluation. Acta Pharmacologica Sinica. 2010; 31: 1625–34;
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|


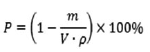
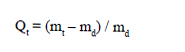 (2)
(2)