J.ophthalmol.(Ukraine).2020;3:3-8.
|
http://doi.org/10.31288/oftalmolzh2020338 Received: 18 February 2020; Published on-line: 24 June 2020 Efficacy of topical N-acetylcysteine as a part of multicomponent treatment for severe dry eye syndrome O.M. Ivanova, G.I. Drozhzhyna, L.F. Troichenko, V.V. Vit, A.B. Abramova, T.D. Lotosh SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine”; Odesa (Ukraine) E-mail: ion2009@ukr.net TO CITE THIS ARTICLE: Ivanova O.M., Drozhzhyna G.I., Troichenko L.F., Vit V.V., Abramova A.B., Lotosh T.D. Efficacy of topical N-acetylcysteine as a part of multi-component treatment for severe dry eye syndrome. J.ophthalmol.(Ukraine).2020;3:3-8.http://doi.org/10.31288/oftalmolzh2020338
Background: According to Tear Film and Ocular Surface Society's Dry Eye Workshop II, the precorneal tear film plays a crucial role in moisturizing the corneal surface and allowing for an optically smooth corneal surface. In addition, as a result of imbalanced secretion of the muco-aqueous phase of the tear film, the mucin layer traps shed epithelial cells, inflammatory cells, and debris to form a mucous clot within the lower conjunctival fornix. Increased mucus viscosity prevents moving mucus away via the punctum, which contributes to the development of severe dry eye syndrome (DES). Our clinical experience argues that tear replacement therapy alone can be not enough in the treatment of severe DES. N-acetylcysteine (NAC), a collagenase inhibitor, has been widely employed clinically as a mucolytic agent. Foreign literature is scant on the use of NAC as an anti-inflammatory, mucolytic, detoxicant and anti-oxidative agent in the treatment of DES. Purpose: To assess the efficacy of topical 5% N-acetylcysteine as a part of multicomponent treatment for severe DES associated with increased mucus production. Material and Methods: We reviewed records of 15 patients examined and treated for severe DES. At baseline, there was biomicroscopic evidence of mucous discharge in the lower conjunctival fornix, corneal epitheliopathy in the form of punctate fluorescein staining, and desquamated epithelium, which was consistent with severe DES. Results: At admission, mean Schirmer’s test value and tear break-up time (TBUT) were 3.4 mm (range, 2 to 6 mm) and 4.2 s (range, 2 to 8 s), respectively, and conjunctival impression cytology revealed focal epithelial degeneration. Topical 5% NAC was administered 4 times daily for 3 months as a part of multicomponent therapy for severe DES. After 3 months of topical 5% NAC, 86.6% of patients noted good tolerance to the drug and improvement of subjective symptoms, and there was biomicroscopic evidence of a reduced amount of mucus in the lower conjunctival fornix and decreased epithelial desquamation. Mean Schirmer’s test and TBUT values increased to 5.0 ± 1.7 mm (mean ± SD) and 6.4 ± 1.7 s, respectively. Conjunctival impression cytology showed the presence of mucoid substance; diffused, differentiated epithelial cells without signs of degeneration; and an adequate superficial conjunctival epithelial cell layer. Conclusion: Topical 5% N-acetylcysteine 4 times daily as a part of multicomponent treatment showed a substantial mucolytic effect for severe DES with apparent thick mucus discharge in the lower conjunctival fornix and resulted in the development of an adequate superficial conjunctival epithelial cell layer. Findings of this study allow us to recommend topical 5% N-acetylcysteine for severe DES patients with increased viscous mucous discharge in the lower conjunctival fornix. Keywords: severe dry eye syndrome, mucin, mucous thread, N-acetylcysteine, mucolytic
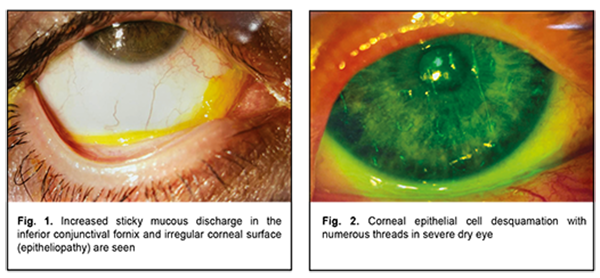
Introduction Ideally smooth, spherical and transparent corneal surface is essential for normal corneal function. This is possible if the corneal epithelial surface is moist, because drying of the anterior epithelium within the area as small as 0.3 μm and at a depth as shallow as 0.5 μm may result in impaired visual perception [1-6]. The precorneal tear film plays a crucial role in moisturizing the corneal surface and allowing for an optically smooth corneal surface; it defends the ocular surface, performs optic and trophic functions, moisturizes eyelids during blinking, and exerts an antibacterial effect. The trilaminar tear film, composed of the lipid, aqueous and mucin layers, normally has a thickness of approximately 2-5.5 μm [7]. The TFOS DEWS II Tear Film Subcommittee recommended a two-phase model of the tear film, which has a lipid layer overlying a muco-aqueous phase [8]. The role of the mucin layer in individuals with normal tear film and patients with dry eye has been discussed in the literature [9-15]. The mucin layer is in direct contact with the epithelial cells of the cornea and conjunctiva, and consists of mucin which is produced mainly by the conjunctival goblet cells, crypts of Henle and glands of Manz; in addition, mucin is synthesized by corneal and conjunctival epithelial cells. The thickness of the tear film mucin layer is 0.02–0.05 μm. At least 20 mucin genes (MUC) have been identified in humans [10, 16-24]. The main function of the mucin layer is converting the corneal surface from a relatively hydrophobic surface to a hydrophilic surface due to binding of polar molecules of mucin with superficial epithelial cells via lipophilic domains and binding of water molecules to hydrophilic domains of mucin molecules. This results in formation of hydrophilic viscous mucin film on hydrophobic membrane of corneal epithelial cells, and the film is capable of keeping on the surface [7, 25-28]. As early as 1975, Ralph [29] emphasized that conjunctival goblet cell loss is a feature of all forms of dry eye disease (DED), and this has been confirmed in later reports, in Sjogren syndrome, ocular cicatricial pemphigoid, alkali burn, radiation keratitis, superior limbic keratoconjunctivitis, trachoma and after LASIK treatment [30-33]. In keeping with this, a decrease in MUC5AC staining has been shown by immuno-fluorescence in conjunctival impression samples from DED patients [30, 34-37]. The mucoaqueous subphase performs a lubricating function between the lids and globe [38]. It also traps shed epithelial cells, inflammatory cells, debris and microorganisms, which are normally removed with tear film from the conjunctival cavity [39, 40]. As a result, these are collected into a mucous thread in the lower conjunctival sac. Increased amount and viscosity of the mucous thread contribute to formation of a mucous clot, which prevents moving mucus away via the punctum, causing foreign body sensation in the eye and increased subjective symptoms of dry eye. Therefore, the tear film is an important component of the visual system. Because changes in homeostasis and function of a tear film layer may result in impairments in other layers, a combined form of dry eye is common [4, 8], which is important to take into account when choosing therapy for dry eye. Although tear replacement, anti-inflammatory and metabolic therapies are common in the treatment of DES, in our clinical experience, these alone can be not enough in the treatment of severe DES due to increased amount and viscosity of the mucous retained within the conjunctival fornix. Since Sheffner [41] first discovered and reported on the mucolytic properties of N-acetylcysteine (NAC), a collagenase inhibitor, in 1963, this drug has been widely employed clinically as a mucolytic agent with low toxicity., In addition, the number of PubMed publications pertaining to this agent which were published in the period from 1963 to this time is as large as 12,727. The list of therapeutic indications for NAC is expanded year by year. The agent is used for impaired mucociliary clearance in airway diseases [42], in the management of cardiac diseases [43, 44] and cancer, transplant procedures [45]; as antidote in poisoning with various substances; and in therapy for HIV infection [46-49]. Foreign literature is scant on the use of NAC as an anti-inflammatory, mucolytic, detoxicant and anti-oxidative agent in severe DED with a highly viscous mucous thread [50]. Because NAC contains free sulfhydryl groups, it breaks disulfide bonds connecting acid mucopolysaccharides, hampers the polymerization of mucoproteins, lowers the viscosity of the mucus, and reduces bacterial adhesion to conjunctival epithelial cells. In addition, it exerts a stimulating effect on mucous cells whose secretion lyses fibrin. Moreover, NAC increases glutathione synthesis and activates detoxication. The anti-inflammatory effect is due to inhibition of formation of free radicals and reactive acid metabolites that cause ocular surface inflammation [50]. A clinical trial demonstrated that oral treatment with mucin depolymerizing agent resulted in decreased ocular dryness symptoms in patients with Sjögren's syndrome. Another study reported that topical NAC had a better effect in reducing subjective symptoms of DED than artificial tears, but had no effect on the objective signs [13, 51]. In addition, several foreign authors reported case series of patients with filamentary keratitis treated with 10% NAC as an adjunct to artificial tear eye drops, cyclosporine and steroids [26, 52]. However, up today, mucolytic, anti-inflammatory, detoxicant and anti-oxidative effects of NAC have not been reflected in indications for use in the management of patients with eye disease. The purpose of the study was to assess the efficacy of 5% N-acetylcysteine in multicomponent treatment of severe dry eye syndrome with increased mucus production. Material and Methods The study protocol (Protocol No. 1, dated February 26, 2019) was approved by the Bioethics Committee of the Filatov Institute. We reviewed records of 15 in-patients (age, 31 to 81 years; mean age, 55.6 years; 5 men and 10 women) examined and treated for severe dry eye disease with increased mucus production at the Department of Corneal Pathology. Patients were included in this study after obtaining informed consent. Patients presented with complaints of photophobia; constant and severe ocular discomfort; foreign body sensation; dryness and tearing; and unstable visual acuity that affected their work capacity. Moderate-to-severe viscous mucous discharge in the conjunctival fornix in the form of mucus threads or clots, corneal epitheliopathy in the form of punctate fluorescein staining, and desquamated epithelium were found in each patient. Patients were diagnosed with severe DES and filamentary keratitis (Figs 1, 2).
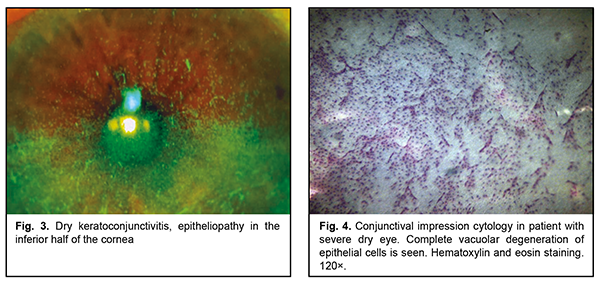
All patients had concurrent diseases. Of these, 10 had rheumatoid arthritis or polyarthritis; one, idiopathic pulmonary arterial hypertension; one, pemphigoid; one, neurotrophic keratitis after radiation therapy for retinoblastoma; one, Reiter's syndrome; and, one patient was after retinal detachment surgery, aphakia and pathologic myopia. All patients underwent an eye examination, including visual acuity measurement, pneumotonometry, perimetry, dry eye tests (Schirmer test and tear break-up time, TBUT), slit lamp biomicroscopy, color photography (HS-5000, Huvitz, Korea), and impression cytology. Impression cytology was performed as follows. After topical anesthesia, a Biopore membrane device (Millicell-CM 0.4 µm PICM 012550, Millipore Corp, Bedford, MA, USA) was applied to the conjunctival surface at the superior temporal conjunctival quadrant, 2-3 mm from the limbus. The filters were fixed in the Nikiforov’s mixture and stained with hematoxylin and eosin. Ready-to-use specimens were covered with polystyrene and examined under a light microscope Jenamed II (Carl Zeiss, Gottingen, Germany) at a magnification of 120×. The cytological pictures, obtained from patients at presentation, formed a basis for comparison. In all patients, topical 5% NAC was administered four times daily for three months as an adjunct to preservative-free hyaluronic acid artificial tears, reparative therapy (Dexpanthenol) and metabolic therapy (vitamins A and E). Because pathological microflora from the conjunctival cavity of patients with severe DES was plated frequently, the conjunctival cavity was sanitized before administration of NAC. Results At baseline, impression cytology of the superficial conjunctival epithelium showed focal degeneration of the epithelial cells as well as scattered groups of discrete cells with no nucleus. In addition, vacuolar degeneration of epithelial cells was seen in 80% of samples. Neither goblet cells nor mucus were seen (Fig. 3). The mean Schirmer test value was 3.4 ± 1.6 mm (mean ± SD), and the mean TBUT test value was 4.2 ± 2.0 s.
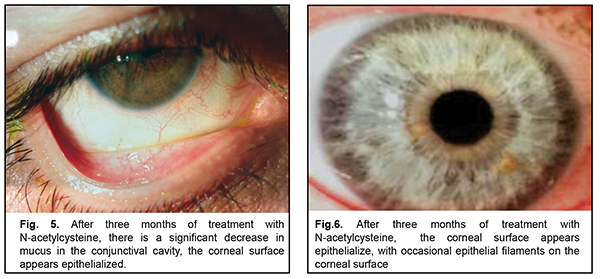
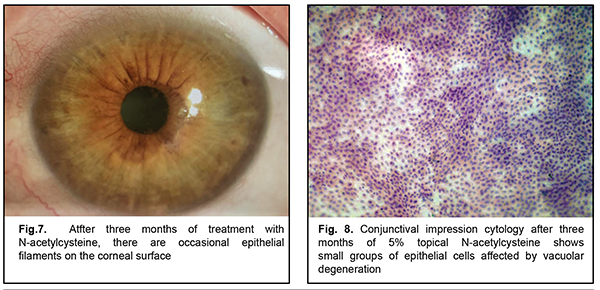
After three months of topical 5% NAC, 13 patients noted good tolerance to the drug, improvement of subjective symptoms (including photophobia and foreign body sensation) and increased periods of stable visual acuity. Two patients (one with cicatricial pemphigoid and one with neurotrophic keratitis after radiation therapy for retinoblastoma) left the study due to increased sensitivity to the agent (ocular mucosa irritation and conjunctival hyperemia). There was biomicroscopic evidence of a reduced amount of mucus in the conjunctival fornix, epithelization of the corneal surface with decreased epithelial desquamation and, however, some punctate fluorescein staining (Fig. 4-5). The mean Schirmer test value changed insignificantly to 5.0 ± 1.7 mm (mean ± SD), and the mean TBUT test value increased to 6.4 ± 1.7 s. After 3-month treatment with 5% NAC, impression cytology showed the presence of meibomian gland secretion and mucoid substance (goblet cell secretion) with the development of an adequate superficial conjunctival epithelial cell layer (Fig. 6).
Discussion After three months of topical 5% N-acetylcysteine 4 times daily, severe dry eye patients with apparent thick mucus discharge in the conjunctival fornix noted good tolerance to the drug and improvement of subjective symptoms and showed reduced amounts of mucus thread, decreased desquamation of epithelium, and epithelized corneal surface. Impression cytology showed an adequate superficial conjunctival epithelial cell layer. Patient selection for topical 5% N-acetylcysteine is still to be discussed, because, in the current study, this treatment was followed by irritation in two patients, likely due to individual sensitivity to the agent. Therefore, findings of this study allow us to recommend topical 5% N-acetylcysteine as a part of multicomponent therapy for severe dry eye patients with increased viscous mucous discharge in the conjunctival fornix.
References 1.Brzheskiĭ VV, Somov EE. [Corneal and conjunctival xerosis (diagnosis, clinical features, and management)]. 2nd ed. St Petersburg: Levsha; 2003. Russian. 2.Golubev SIu, Kuroiedov AV. [On the selection of economically efficacious agent for the prevention and treatment of dry eye syndrome]. Sindrom sukhogo glaza. 2002;3:12-4. Russian. 3.Egorov EA, Basinskii SN. [Clinical lectures on ophthalmology]. Moscow: GEOTAR; 2007. Russian. 4.Polunin GS, Polunina EG. [From dry eye to team film disease]. Oftalmologiia. 2012;9(2):4-7. Russian. 5.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II Pathophysiology report. Ocul Surf. 2017;15:438-510. 6.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017 Jul;15(3):334-365. 7.Vit VV. [Disorders of the eye, ocular adnexa and orbit], Vol. 1. Odesa: Astroprint; 2019. Russian. 8.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017 Jul;15(3):276-283. 9.Cheng K. H., Spanjaard L., Rutten H. et al. Immunoglobulin A antibodies against Pseudomonas aeruginosa in the tear fluid of contact lens wearers. Invest Ophthalmol Vis Sci. 1996. Vol. 37:2081-2088. 10.Little SA, Bruce AS. Postlens tear film morphology, lens movement and symptoms in hydrogel lens wearers. Ophthal Physiol Opt. 1994 Jan;14(1):65-9. 11.Lowther GE. Dryness, Tears and Contact Lens Wear. Boston: Butterworth-Heinemann; 1997. 12.Ohashi Y, Motokura M, Kinoshita Y. Presence of epidermal growth factor in human tear. Invest Ophthalmol Vis Sci. 1989;30:1879–82. 13.Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016 Feb 23;2:CD009729. 14.Roth HW. Studies on the etiology and treatment of giant papillary conjunctivitis in contact lens wearers. Contactologia. 1991;13E:55-60. 15.Young G, Efron N. Characteristics of the pre-lens tear film during hydrogel contact lens wear. Ophthal Physiol Opt. 1991 Jan;11(1):53-8. 16.Aragona P, Di Pietro R, Spinella R, Mobrici M. Conjunctival epithelium improvement after systemic pilocarpine in patients with Sjogren's syndrome. Br J Ophthalmol. 2006;90(2):166-70. 17.Hamano H, Hori M, Hamano TA. A new method for measuring tears. CLAO J. 1983 Jul-Sep;9(3):281-9. 18.Somov EE. [Etiopathogenetic basic of dry eye syndrome and principles for an approach to treating the disease]. In: [Proceedings of Opthalmic Conference, Neva Horizons-2010]. Vol. 2. St Petersburg. October 15-16, 2010. Russian. 19.Downie LE, Keller PR. A pragmatic approach to dry eye diagnosis: evidence into practice. Optom Vis Sci. 2015;92(12):1189-97. 20.Ibrahim OM, Dogru M, Kawashima S, Wakamatsu TH, Tsubota K, Fujishima H. Visante optical coherence tomography and tear function test evaluation of cholinergic treatment response in patients with Sjogren syndrome. Cornea. 2013;32(5):653-7. 21.Petrone D, Condemi JJ, Fife R, Gluck O, Cohen S, Dalgin P. A double-blind, randomized, placebo-controlled study of cevimeline in Sjogren's syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002;46(3):748-54. 22.Wegener AR, Meyer LM, Schönfeld CL. Effect of viscous agents on corneal density in dry eye disease. J Ocul Pharmacol Ther. 2015 Oct;31(8):504-8. 23.Jordan A, Baum J. Basic tear flow: does it exist? Ophthalmology. 1980 Sep;87(9):920-30. 24.Lemp MA. The mucin-deficient dry eye. Int Ophthalmol Clin. Int Ophthalmol Clin. 1973 Spring;13(1):185-9. 25.Somov EE, Brzheskiĭ VV. [Tear. Physiology, examination methods and clinical features]. St Petersburg: Nauka; 1994. Russian. 26.Albietz J, Sanfilippo P, Troutbeck R, Lenton LM. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci. 2003;80(6):420-30. 27.Inatomi T, Spurr-Michaud SJ, Tisdale AS, Gipson lK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995 Aug;36(9):1818-27. 28.Inatomi T, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Feidman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1684-92. 29.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14(4):299-302. 30.Abreau K, Callan C, Kottaiyan R, Zhang A, Yoon G, Aquavella JV, et al. Temperatures of the Ocular Surface, Lid, and Periorbital Regions of Sjogren's, Evaporative, and Aqueous-Deficient Dry Eyes Relative to Normals. Ocul Surf. 2016;14:64-73. 31.Baudouin C, Messmer EM, AragonaJ P, Geerling G, Akova YA, Benitez-Del-Castillo J, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300-6. 32.Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease. A better indicator than tear mucin. Arch Ophthalmol. 1983;101:1284-7. 33.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223-35. 34.Corrales RM, de Paiva CS, Li DQ, Farley WJ, Henriksson JT, Bergmanson JP, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52:3492-9. 35.Pflugfelder SC, Stern ME. Mucosal environmental sensors in the pathogenesis of dry eye. Expert Rev Clin Immunol. 2014;10:1137-40. 36.Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013 Sep;32(9):1211-8. 37.Zhang J, Yan X, Li H. Analysis of the correlations of mucins, inflammatory markers, and clinical tests in dry eye. Cornea. 2013 Jul;32(7):928-32. 38.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379-88. 39.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31:271-85. 40.Norn MS. Dead, degenerate, and living cells in conjunctival fluid and mucous thread. Acta Ophthalmol (Copenh). 1969;47:1102-15. 41.Sheffner AL. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann NY Acad Sci. 1963 Mar 30;106:298-310. 42.Sakharov GM, Antonov NS. [Rendering care regarding tobacco dependence in therapeutic practice: A tutorial for physicians]. Moscow; 2010. Russian. 43.Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis. Eur J Cardiothorac Surg. 2009 Mar;35(3):521-7. 44.Kwok CS, Pang CL, Yeong JK, Loke YK. Measures used to treat contrast-induced nephropathy: overview of reviews. Br J Radiol. 2013 Jan; 86(1021):20120272. 45.Inci I, Erne B, Arni S, Jungraithmayr W, Inci D, Hillinger S, et al. Prevention of primary graft dysfunction in lung transplantation by N-acetylcysteine after prolonged cold ischemia. J Heart Lung Transplant. 2010;29(11):1293–301. 46.Borges-Santos MD, Moreto F, Pereira PC, Ming-Yu Y, Burini RC. Plasma glutathione of HIV⁺ patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition. 2012 Jul;28(7-8):753-6. 47.Goossens N, Ditisheim S, Lanthier N, Spahr L, Hadengue A. [Alcoholic steatohepatitis: what's new in 2012?]. Rev Med. Suisse. 2012 Sep 5;8(352):1646-8, 1650-1. French. 48.Grant LM, Rockey DC. Drug-induced liver injury. Curr Opin Gastroenterol. 2012;28:198–202. 49.Müller, Svardal AM, Nordoy I, Berge RK, Aukrust P, Frøland SS. Virological and immunological effects of antioxidant treatment in patients with HIV infection. Eur J Clin Invest. 2000 Oct;30(10):905-14. 50.Jones L. TFOS DEWS II Management and Therapy Report. Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. Ocul Surface. 2017 Jul;15(3):575-628. 51.Pokupec R, Petricek I, Sikic J, Bradic M, Popovic-Suic S, Petricek G. Comparison of local acetylcysteine and artificial tears in the management of dry eye syndrome. Acta Med Croat. 2005;59:337-40. 52.Pentapati M, Shah S. Filamentary keratitis: A case series. Int J Scientific Res Pub. 2015 Mar;5(3):1-7. 53.Ichikawa Y, Tokunaga M, Shimizu H, Moriuchi J, Takaya M, Arimori S. Clinical trial of ambroxol (Mucosolvan) in Sjögren's syndrome. Tokai J Exp Clin Med. 1988 Aug;13(3):165-9. 54.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetylcysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38-44.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|