J.ophthalmol.(Ukraine).2020;2:50-55.
|
http://doi.org/10.31288/oftalmolzh202025055 Received: 21 January 2020; Published on-line: 30 April 2020 System analysis of factors in the pathogenesis of drusen formation in AMD S.Yu. Mogilevskyy, Dr Sc (Med), Prof.; Kh.V. Kovalchuk, Post-grad Student Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) E-mail: kris.ophthalm@gmail.com TO CITE THIS ARTICLE: Mogilevskyy SYu, Kovalchuk KhV. System analysis of factors in the pathogenesis of drusen formation in AMD. J.ophthalmol.(Ukraine).2020;2:50-55. http://doi.org/10.31288/oftalmolzh202025055
Background: Age-related macular degeneration (AMD) is a major cause of visual loss in elderly individuals. However, the involvement of cell receptors in the initiation of retinal disorder in AMD has not been duly reflected in the literature. The absence of system analysis of pathogenetic mechanisms of drusen formation restricts the possibilities for researchers to develop methods of treatment and prevention of early AMD. Purpose: To carry out system analysis of novel factors in the pathogenesis of drusen formation in AMD. Material and Methods: This prospective study included 109 patients (186 eyes) with intermediate stage of dry AMD. All patients received a detailed eye examination before and in the course of treatment. We used receptor ligands (specifically, adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine, platelet activation factor (PAF), adrenaline and isadrine) to examine the functional activity of platelet receptors. Platelet aggregation was assessed by the turbidimetric method. The effect of platelet reactivity on the formation of drusen in the RPE was also assessed. Results: The comparison of Group 1 patients with and without drusen (subgroups A and B, respectively) versus controls with regard to platelet reactivity indicated that hyperreactivity of alpha-2 adrenoreceptors reflected early accumulation of metabolite residues in RPE cells and appearance of drusen in the retina. An increase in drusen numbers in the RPE in Group 2 (subgroup C) patients with early dry AMD compared to subgroup B with was reflected by increased activity of P2X purine receptors (р < 0.05) and A2A adenosine receptors (р < 0.001). In group 3 (subgroup E) patients with intermediate stage of dry AMD, an increase in the size of drusen was accompanied by changes in activity of the four examined platelet receptors, with increases in sensitivity of alpha-2 adrenoreceptor, P2X and PAF receptors, and a decrease in sensitivity of beta-2 adrenoreceptor. Conclusion: Platelet receptors reactivity reflected the effect of factors in the pathogenesis of AMD on drusen formation, and, therefore, can be used for prediction of the risk of disease progression. Keywords: age-related macular degeneration, platelet receptors, retinal pigment epithelium, drusen formation
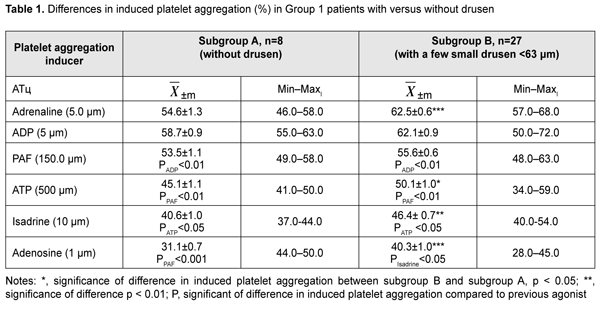
Introduction Age-related macular degeneration (AMD) is a major cause of visual loss in elderly individuals [1]. The average annual incidence in individuals aged 67 years or older has been estimated to be approximately 3% [2]. Age, sex, diet, body mass index (BMI), presence of hypertonia, level of high density lipoprotein-like particles (HDLP), cumulative sunlight exposure, and smoking influence the development and progression of AMD [3]. However, the involvement of cell receptors in the initiation of retinal disorder in AMD has not been duly reflected in the literature. Lipid accumulation in the form of drusen or linear deposits in Bruch’s membrane is accompanied by impaired transport of substances and oxygen from blood to retinal cells as well as translocation of catabolites to choriocapillaris [4]. The absence of system analysis of pathogenetic mechanisms of drusen formation restricts the possibilities for researchers to develop methods of treatment and prevention of early AMD. The purpose of the study was to carry out system analysis of novel factors in the pathogenesis of drusen formation in AMD. Material and Methods This prospective study included 109 patients (186 eyes) with intermediate stage of dry AMD. AMD stage was classified according to the Age-Related Eye Disease Study Research Group (AREDS) staging system. All patients provided informed consent before entering the study. Patients were divided into groups for analysis of intensity of drusen formation in RPE cells. Group 1 (n=35) was composed of patients with no AMD (AREDS stage 1). These were divided into two subgroups, A (n=8), with no drusen and B (n=27), with a few small drusen (<63 ?m). Group 2 (n=36) was composed of patients with early AMD (AREDS stage 2). These were divided into two subgroups, C (n=19), with numerous small drusen and several intermediate drusen (63 ?m to 124 ?m) and D (n=17), with numerous small drusen, several intermediate drusen and mild changes in retinal pigment epithelium (RPE). Group 3 (n=38) was composed of patients with intermediate dry AMD (AREDS stage 3). These were divided into two subgroups, Е (n=21), with numerous intermediate drusen and at least one large druse (?125 ?m), and F (n=17), with numerous intermediate drusen and geographic atrophy not involving the central fovea. Ocular history was collected, visual acuity was assessed, and tonometry, biomicroscopy, ophthalmoscopy and optical coherence tomography (OCT) were performed. Fluorescein angiography was conducted if latent neovascularization was suspected. Platelets were isolated by centrifugation of patient’s citrated peripheral blood and used to assess the functional activity of receptors. We used agonists of the receptors involved into AMD pathogenesis, specifically, adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine (ligands of Р2Х, Р2Y, and А2А purine receptors, respectively), platelet activation factor (PAF), and adrenaline and isadrine (ligands of alpha-2 and beta-2 adrenoreceptors, respectively). Activity of adenosine A2A- receptors and beta-2 adrenoreceptors was investigated through incubation of ADP with either adenosine or isadrine, and calculated as the difference between ADP-induced platelet aggregation and residual platelet aggregation for incubation of ADP with either adenosine or isadrine. The agonists were obtained from Sigma (St. Louis, MO) and used in EC50 concentrations to produce 50% ± 5 % of the maximum rate of aggregation in 10 healthy volunteer donors without changes in the fundus. Platelet aggregation was assessed by the turbidimetric method at the Research Institute for Experimental and Clinical Medicine (Bohomolets National Medical University) headed by Prof. L.V. Natrus. Statistical analysis was performed using Medcalc Software. The mean (Х) and standard error of the mean (m) were calculated for the groups. Statistical comparisons between the two groups were performed using a Student's t-test in the case of quantitative, normally distributed variables and a Wilcoxon rank-sum test in the case of quantitative, not normally distributed variables. Differences were considered statistically significant at p < 0.05. Results and Discussion Table 1 presents percentage differences in platelet aggregation between Group 1 patients with and without drusen. We found platelet hyperreactivity to ADP and adrenaline in Group 1 patients with and without drusen versus controls.
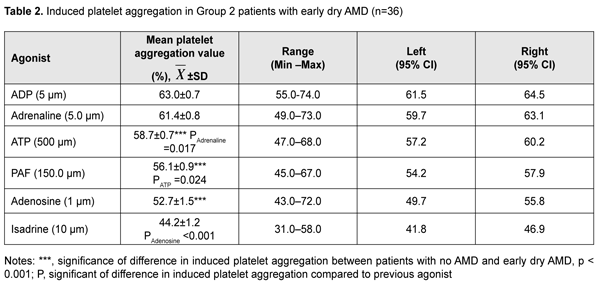
Platelet responses to PAF, ATP and isadrine corresponded to the reference range for normal platelet response, whereas platelet responses to adenosine corresponded to platelet hyporesponse. Activity of alpha-2 adrenoreceptors was comparable with that of Р2Y-receptors and was 12.4% (р < 0.01), 24.7% (р < 0.001), 34.7% (р < 0.001), and 55.1% (р < 0.001) higher than that of PAF, Р2Х, beta-2 and А2А receptors, respectively. Therefore, the comparison of subgroups A and B for functional activity of receptors could indicate that hyperreactivity of alpha-2- adrenoreceptors is associated with early accumulation of metabolite residues of RPE cells and appearance of drusen in the retina. Therefore, the in vitro platelet study enabled not only to analyze the functional activity of the six receptors reflecting the effect of systemic factors in the pathogenesis of AMD on blood cells, but also to clarify a major mechanism that triggered changes in functional activity of platelets. In Group 2 patients with early AMD (mean age, 71.6 ± 1.8 years), four receptors (Р2Х- and Р2Y- purine receptors, alpha-2 adrenergic receptor and PAF-receptor) reflected platelet hyperreactivity; normal response was characteristic for A2A-receptor (95%CI, 49.7%–55.8%), and hyporeactivity, for beta-2 adrenoreceptor (Table 2). Platelet response to ADP was comparable with that to adrenaline, and was 7.3% (р < 0.001), 12.3% (р < 0.001), 19.5% (р < 0.001), and 42.5% (р < 0.001) higher than that to ATP, PAF, adenosine, and isadrine, respectively. The comparison between patients with no AMD and those with early dry AMD with respect to platelet activity allowed us to conclude that an increase in activity of two P2X receptors (by 20%; р < 0.001) and A2A receptors (by 38%; р < 0.001) reflected the initiation of AMD development. The presence of a positive relationship of old age of patients with AMD (but not old age of patients without AMD) with increased reactivity of PAF- and alpha-2 adrenergic receptors indicated that the development of inflammation (resulting from the effect of activated white blood cells on platelets) and activation of the sympathoadrenal system (SAS) are major risk factors for early AMD. In addition, adrenaline may be a trigger of increased reactivity of PAF- receptors, that is, the SAS initiates chronic inflammation. The effect of activated white blood cells on platelets was limited (i.e., signaling of PAF-receptors was inhibited) by maintained normal reactivity of beta-2-adrenoreceptors. Unilateral PAF-induced platelet aggregation and ADP/ ATP-induced platelet aggregation confirmed platelet stimulation by white blood cells. This was accompanied by activation of purine secretion by platelets and stimulation of Р2Х and Р2Y-receptors of white blood cells by active platelets.
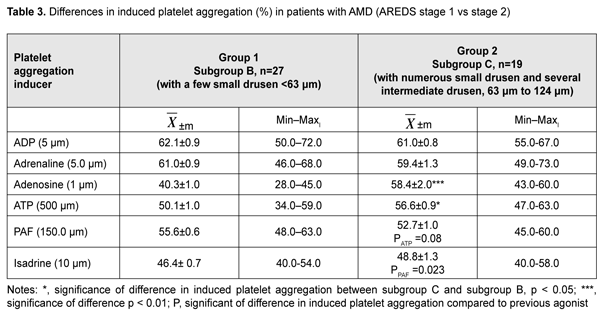
The comparison between subgroups B (AREDS stage 1, a few small drusen) and C (AREDS stage 2, numerous small drusen) allowed for analysis of cases with increased number of drusen (Table 3). These changes in drusen formation may, to some extent, indicate accumulation of cell metabolism products (including lipid metabolites) in the RPE. Hyperreactivity of two platelet receptors (P2Y purine receptors and alpha-2 adrenoreceptor) was noted with the progression of drusen formation. In subgroup C, activity of P2Y purine receptors was 13% higher (р < 0.01), and activity of A2A-purine receptors was 44.9% higher (р < 0.001), compared to subgroup B. Activity of PAF receptors and beta-2 adrenoreceptors in subgroup C was not changed and was within the range of normal reactivity.
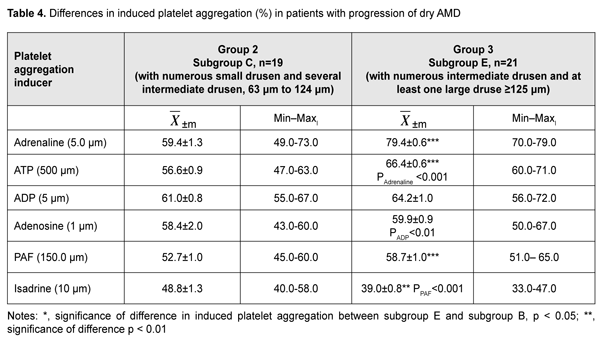
Therefore, first, there was hyperreactivity of four receptors (alpha-2 adrenoreceptor, P2Y- and P2X-purine receptors, and A2A-receptor), whereas reactivity of two other receptors (PAF-receptor and beta-2 adrenoreceptor) conformed to the range of normal platelet reactivity (50.0 ± 5.0%). Second, activity of P2Y-receptors was comparable to that of alpha-2 adrenoreceptors and A2A-receptors, and was 7.8% (р = 0.001), 15,8% (р < 0.001) and 25% (р < 0.001) higher than that of P2X-purine receptors, PAF-receptors, and beta-2 adrenoreceptors, respectively. The observed hyperreactivity of P2X-and P2Y-purine receptors and alpha-2 adrenoreceptors is a result of the interaction of these receptors. In particular, there were negative pairwise correlations between activities of P2X-and A2A-receptors (r = -0.420; р < 0.05); Р2Y- and А2А- receptors (r=-0.714; р<0.05); alpha-2- and beta-2-adrenoreceptors (r=-0.610; р<0.05); and PAF-receptors and А2А-receptors (r=-0.585; р<0.05). The found correlations reflected the function of compensatory mechanisms with involvement of adenosine receptors and beta-2 adrenoreceptors, which provided not only limitation for platelet aggregation, but also anti-inflammatory effect (inhibition of platelet response to PAF secretion by white blood cells). Therefore, an increase in drusen numbers in the RPE is accompanied by increased reactivity of P2X purine receptors (р < 0.05) and A2A adenosine receptors (р < 0.001). The intermediate stage of dry AMD is important for the analysis, since at this stage, new changes in RPE cells take place, which was manifested by an increase in drusen size in subgroup E. In addition, we found that, in the presence of numerous intermediate-size drusen, P2X, P2Y and A2A purine oreceptors and PAF-receptors reflected platelet hyperreactivity; whereas hyporeactivity was characteristic for beta-2 adrenoreceptor (Table 4). In subgroup E, platelet responses to adrenalin, ATP, adenosine and PAF were 33.7% (р < 0.001), 17.3% (р < 0.001), 32.5% (р < 0.001) and 11.4% higher, respectively, platelet response to isadrine was 20.1% (р < 0.01) lower compared to subgroup C, and platelet responses to ADP and adenosine were similar to those in subgroup C. Thus, with increased changes in metabolism in the RPE and increased drusen formation, activity of the four examined platelet receptors changed. Specifically, sensitivity of alpha-2 adrenoreceptor, P2X and PAF receptors increased, whereas sensitivity of beta-2 adrenoreceptor decreased. Therefore, reactivity of platelet receptors reflected the effect of factors of AMD pathogenesis on drusen genesis, and, thus, can be utilized for predicting the risk of AMD progression. AMD progression has been reported to be associated with reduced capacity of RPE cells for autophagy [5]. Autophagy plays an important role in the protection of RPE cells from oxidative stress and lipofuscin accumulation; impaired autophagy increases oxidative stress and contributes to the development of the AMD. Oxidative stress inhibits RPE cell phagocytosis with activation of cAMP-activated protein kinase [6]. Similar effect was reproduced by direct enzyme stimulation with 5-aminoimidazole-4-carboxamide riboside (AICAR). Modified mice studies demonstrated that RPE cell phagocytosis was controlled by alpha-2 adrenoreceptors and activation of these receptors promoted inhibition of phagocytosis in oxidative stress; this effect was associated with increased phosphorylation of acetyl-CoA carboxylase. ATP levels are known to be decreased in RPE cell phagocytosis, but the ecto-5’-nucleotidase inhibitor beta-methylene ADP prevented this effect [7]. One could hypothesize that autophagy inhibition was mediated by non-elucidated purine receptors. Ecto-5’ nucleotidase activity on the apical membrane of RPE cells was examined in 2006, and it was found that stimulation of alpha-2 adrenoreceptors inhibited enzyme activity, resulting in increases in adenosine levels and activity of alpha-2 receptors, while a decrease in subretinal adenosine levels contributed to increased phagocytosis of rod outer segments [8]. Subsequently, it was found that blocking adenosine A2A receptors in human microglia increases the clearance of apoptotic photoreceptors. Therefore, it is increased activity of alpha-2 adrenoreceptors and adenosine A2A receptors that are risk factors of drusen genesis [9]. It has been established previously that stimulation of purine receptor P2X7 increased pH levels in lysosomes in cultivated human RPE cells, which resulted in increased concentrations of cytoplasmic Ca2+ and decreased approach to the active site of cathepsin D, leading to lysosomal dysfunction [10]. Therefore, endogenous autostimulation of the P2X receptors could hamper the removal of oxidized metabolites from cells including lipids, confirming the possible role of excessive ATP stimulation of RPE cells in lipid accumulation. Other studies confirmed that, in release of ATP from RPE cells, autocrine P2X7 stimulation by ATP is possible and targeted at increases in lysosomal pH and lipofuscin production [11]. Adenosine may reactivate lysosomal function through A2A receptors and decrease lipid oxidation. Therefore, in RPE cells, there is an adaptive mechanism that maintains the balance between extracellular ATP and adenosine, and, in this way, can modulate lysosomal pH and the rate of production of lipofuscin.
Conclusion First, our platelet in vitro study enabled (1) the analysis of functional activity of receptors that reflect the effect of systemic factors in the pathogenesis of AMD on target cells and (2) elucidation of major mechanisms that trigger changes in RPE. Second, the appearance of drusen in early AMD as a possible consequence of accumulation of metabolic products in the RPE is associated with hyper-response of platelet alpha-2 adrenoceptors. Progression of drusen formation was accompanied by increased activity of P2X receptors and PAF receptors and decreased activity of beta2 adrenoreceptors. Platelet receptors reactivity reflected the effect of factors in the pathogenesis of AMD on drusen formation, and, therefore, can be used for prediction of the risk of disease progression. Finally, increased activity of P2X and A2A receptors, and the presence of a positive relationship between elderly age of patients and increased platelet PAF receptor and alpha-2 adrenoceptor reactivity may indicate that the pathophysiological processes (like retinal hypoxia, ischemia and inflammation) associated with these changes may be major factors in the development of AMD.
References 1.Brandl C, Br?cklmayer C, G?nther F, Zimmermann ME, K?chenhoff H, Helbig H, Weber BHF, Heid IM, Stark KJ. Retinal Layer Thicknesses in Early Age-Related Macular Degeneration: Results From the German AugUR Study. Invest Ophthalmol Vis Sci. 2019; 60(5):1581-94. 2.Jonasson F, Fisher DE, Eiriksdottir G, Sigurdsson S, Klein R, Launer LJ, Harris T, Gudnason V, Cotch MF. Five-year incidence, progression, and risk factors for age-related macular degeneration: the age, gene/environment susceptibility study. Ophthalmology. 2014; 121:1766–72. 3.Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006; 90:75–80. 4.Algvere PV, Kvanta A, Seregard S. Drusen maculopathy: a risk factor for visual deterioration. Acta Ophthalmol. 2016;94(5):427-33. 5.Hyttinen JMT, B?asiak J, Niittykoski M, Kinnunen K, Kauppinen A, Salminen A, Kaarniranta K. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells-Implications for age-related macular degeneration (AMD). Ageing Res Rev. 2017; 36:64-77. 6.Qin S, De Vries GW. Alpha2 but not alpha1 AMP-activated protein kinase mediates oxidative stress-induced inhibition of retinal pigment epithelium cell phagocytosis of photoreceptor outer segments. J Biol Chem. 2008; 283(11):6744-751. 7.Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001;534(Pt 1):193-202. 8.Reigada D, Zhang X, Crespo A, Nguyen J, Liu J, Pendrak K, Stone RA, Laties AM, Mitchell C. Stimulation of an alpha1-adrenergic receptor downregulates ecto-5' nucleotidase activity on the apical membrane of RPE cells. Purinergic Signal. 2006;2(3):499-507. 9.Madeira MH, Rashid K, Ambr?sio AF, Santiago AR, Langmann T. Blockade of microglial adenosine A2A receptor impacts inflammatory mechanisms, reduces ARPE-19 cell dysfunction and prevents photoreceptor loss in vitro. Sci Rep. 2018;8(1):2272. 10.Guha S, Liu J, Baltazar G, Laties AM, Mitchell CH. Rescue of compromised lysosomes enhances degradation of photoreceptor outer segments and reduces lipofuscin-like autofluorescence in retinal pigmented epithelial cells. Adv Exp Med Biol. 2014;801:105-11. 11.Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, M?ller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270-9.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|