J.ophthalmol.(Ukraine).2020;2:65-69.
|
http://doi.org/10.31288/oftalmolzh202026569 Received: 30 January 2020; Published on-line: 30 April 2020 Temperature of the ocular surface in the projection of the ciliary body in rabbits Dorokhova O., Zborovska O., Meng Guanjun, Zadorozhnyy O. Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) E-mail: dorochovaa@gmail.com TO CITE THIS ARTICLE: Dorokhova O, Zborovska O, Meng Guanjun, Zadorozhnyy O. Ocular surface temperature in the projection of the ciliary body in rabbits. J.ophthalmol.(Ukraine).2020;2:65-69. http://doi.org/10.31288/oftalmolzh202026569
Background: Studies on local body temperatures improve the potential for analysis of biological processes in organs and tissues of the body. A study on changes in ocular surface temperature in an animal model of non-infectious uveitis for objectively assessing ocular inflammation is planned for the future. Purpose: To determine normal temperatures of the ocular surface in the projection of the ciliary body in rabbits. Material and Methods: Temperature of the ocular surface in the projection of the pars plana was measured in 42 Chinchilla rabbits using a thermoelectric device. Results: There was no significant difference in mean temperature between the nasal ocular surface and the temporal ocular surface in the projection of the ciliary body (34.13 °С (SD=1.45) against 34.09 °С (SD=1.48), p = 0.57). In addition, there was a very weak correlation of room temperature with body temperature and ocular surface temperature. Mean ocular surface temperature for the right eye was 34.1°С (SD=1.43), and for the left eye, 34.2°С (SD=1.43), but the difference in this measure between the right and left eyes was not significant (р = 0.27). Conclusion: There was no significant difference in the temperature of the ocular surface in the projection of the ciliary body between the right and left eyes in the absence of pathological changes. The mean temperature of the ocular surface in the projection of the ciliary body in intact rabbits was 34.1°С (SD=1.4). Temperature of the ocular surface in the projection of the ciliary body in rabbits is characterized by autonomic thermoregulation and is relatively stable at small environmental temperature variations. The pattern of heat exchange in rabbit’s ocular surface allows for modeling of unilateral ocular pathological processes that would have a change in local body temperature in the projection of the ciliary body as an objective marker. Keywords: objectivization of inflammation assessment, ocular surface temperature, ciliary body, thermoelectric device
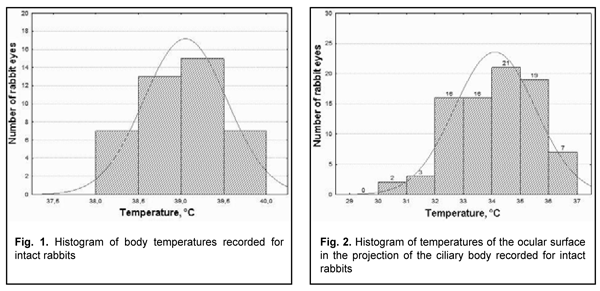
Introduction At least 150 conditions are known to be associated with intraocular inflammation. Uveitis is a pathogenetically complex and potentially blinding intraocular inflammatory condition whose etiology remains elusive and whose treatment continues to be a challenge despite recent advances [1-4]. The severity of ocular inflammatory injury depends on the activity and type of inflammation. The decision on the amount of immune suppression and duration of immune suppression therapy to be administered should be based on the assessment of inflammation severity. Therefore, the clinician’s decision on the treatment strategy and duration of treatment should be guided by quantitative assessment of inflammation. Currently, most studies use Standardization of Uveitis Nomenclature (SUN) and National Institutes of Health (NIH) classification schemes for assessing uveitis activity [5, 6]. However, one of the major disadvantages of these classification schemes and their methodologies used for detecting inflammation in the aqueous and vitreous is subjective grading. A study demonstrated disparity in slit lamp flare readings between clinical graders in clinical grading in 35% of eyes [7, 8]. A change in the degree of activity of the process is an important index for assessing the efficacy of therapy in patients with uveitis [9]. Laser flare-cell photometry provides an automated technique to quantify the assessment of cells and protein levels ("flare") in the aqueous humor objectively, and it has been used in a variety of research and clinical situations to assess even subclinical anterior segment inflammation [10-14]. A number of factors (the degree of mydriasis, cataract, and/or posterior synechiae) can affect the results of laser flare-cell photometry. In addition, the broad application of the technique is limited by high cost of laser flare-cell photometer and the absence of consensus on the clinical value of the methodology [15, 16]. Studies on local body temperatures improve the potential for analysis of biological processes in organs and tissues of the body. Heat energy is constantly produced in the body and the amount of heat energy produced depends on the intensity of metabolic and inflammatory processes as well as state of circulation [17]. It is at the local level associated with inflammation focus that the signs of inflammation (hyperemia, locally increased temperature, edema, pain and impaired function of the damaged organ) are revealed, the mechanisms behind these signs being the molecular and cellular inflammatory mechanisms [18]. Researchers have been searching for simple, inexpensive and reliable methods for objective assessment of intraocular inflammation for years. Since measurement reproducibility is important, especially in clinical trials, recently, researchers have been trying to gradually change subjective inflammation assessment methods in favor of objective inflammation assessment methods. Local measurements of temperature responses have been successfully used in other fields of medicine, and seem promising for objective assessment of ocular inflammation. Research on changes in ocular surface temperature in an animal model of non-infectious uveitis is planned for the future to determine whether it is possible to objectively assess inflammation using ocular surface temperature, and to match these changes with pathoanatomical changes. With this in mind, we have to determine normal temperatures using precise measurement equipment, and to determine whether rabbit eyes are suitable for this purpose. Therefore, the purpose of the study was to determine normal temperatures of the ocular surface in the projection of the ciliary body in rabbits. Material and Methods Forty-two Chinchilla rabbits (84 eyes; weight, 2.5-3.0 kg) were included in this study. Prior to baseline, they were under quarantine for two weeks. The animals were housed under conventional vivarium conditions, and fed and watered conventionally. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Temperature of the ocular surface in the projection of the ciliary body (2-3 mm from the limbus) was measured through direct contact of the probe tip and nasal or temporal scleral conjunctiva 15 minutes after both eyes received a drop of proxymetacaine HCl (0.5%) for topical anesthesia. Real-time temperature measurements were recorded every 4 seconds. Measurements were performed at least three times in each of the compartments. Rectal temperature and room temperature were also recorded. Studies were performed under minimal indoor air velocity. A thermoelectric device [19] developed within the framework of the partnership agreement between the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring ocular surface temperature. The device allows for measurements every 4 seconds within a temperature range of -10°С to + 120°С with a measurement error of ± 0.08°С. Data is presented as mean ± standard deviation (SD). Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ? 0.05 was assumed. Results and Discussion The ambient room temperature was between 18 °С and 23 °С. Body temperature in intact rabbits followed a normal distribution (K-S d = 0.08, p>0.2) (Fig. 1).
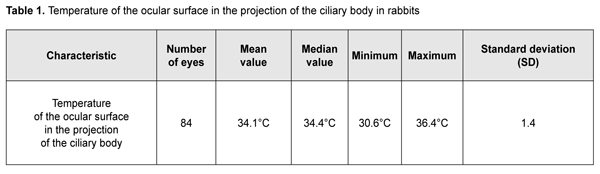
Mean temperature of the nasal ocular surface in the projection of the ciliary body was 34.13 °С (SD=1.45), and mean temperature of the temporal ocular surface was 34.09 °С (SD=1.48). Since there was no statistically significant difference (р=0.57) between these mean values, for convenience of subsequent calculations, we were taking into account mean temperature of the ocular surface in the projection of the ciliary body. Ocular surface temperature in intact rabbits followed a normal distribution (K-S d = 0.09, p>0.2) (Fig. 2). Temperature of the ocular surface in the projection of the ciliary body ranged from 30.55 °С to 36.35 °С, with a mean value of 34.11 °С (SD=1.421) (Table 1).
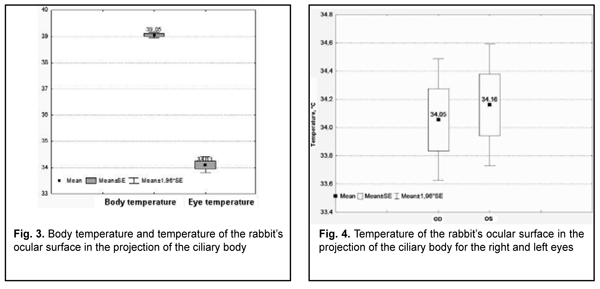
There was a mild correlation between room temperature and body temperature (r=0.30; р=0.05). In addition, there was a very weak but not significant correlation (r=0.02; p = 0.9) between room temperature and temperature of the ocular surface in the projection of the ciliary body. Moreover, there was a very weak but not significant negative correlation (r=-0.04; p = 0.82) between body temperature and temperature of the ocular surface in the projection of the ciliary body. We found a significant positive correlation between temperatures of temporal and nasal ocular surface (r = 0,895, p = 0.0001). It seemed strange that correlation of room temperature with body temperature and temperature of the ocular surface in the projection of the ciliary body was weak and not significant. For this reason, we additionally assessed whether there was a significant difference in mean temperature between the body and ocular surface at different room temperatures. For this purpose, we separated room temperatures into two categories: first, those below 20°С, and, second, those above 20°С. At room temperatures below 20°С, the mean body temperature was 38.97°С, and at room temperatures above 20°С, the mean body temperature was 39.17°С, but the difference was not significant (р = 0.19). In addition, at room temperatures below 20°С, the mean temperature of the ocular surface in the projection of the ciliary body was 34.3°С, and at room temperatures above 20°С, it was 33.69°С, but the difference was also not significant (р = 0.17). A rabbit study by Schwartz [20] assessed the effect of environmental temperature on temperatures of the corneal surface, conjunctiva, intraocular media and orbit. Three ranges of environmental temperatures were used: 22.0 to 27.5 °С, 15.9 to 17.6°С, and 2.2 to 4.4 °С. Rectal temperature was noted to decrease with a decrease in air temperature, and there was a linear relationship between a decrease in corneal surface and conjunctival temperatures and a decrease in environmental temperature. In addition, the decrease in the temperature of the cornea was five times as large as that in the temperature of the inferior conjunctival fornix, which was likely due to the avascularity of the cornea. Moreover, temperatures of all intraocular structures were noted to decrease with a decrease in environmental temperature [20]. Others reported similar findings on the effect of environmental factors on corneal surface temperature in rabbits. In addition, a linear relationship between corneal surface temperature and air temperature at zero air speed has been reported [21, 22]. Our findings of weak and not significant correlation of environmental temperature with body temperature and temperature of the ocular surface in the projection of the ciliary body could be explained by the fact that, as opposed to the above studies, the environmental temperature in the current study varied within a small range. Therefore, for this small environmental temperature range, the autonomic thermoregulation may ensure relative stability in the temperature of the ocular surface in the projection of the ciliary body. The temperature of the ocular surface in the projection of the ciliary body was 5°С below body temperature, and this difference was significant (р=0.0001) (Fig. 3).
Mean ocular surface temperature for the right eye was 34.1°С (SD=1.43), and for the left eye, 34.2°С (SD=1.43). There was a 0.11°С difference in ocular surface temperature between the right and left eyes, but this was not significant (р = 0.27) (Fig. 4). The absence of significant difference in the temperature of the ocular surface in the projection of the ciliary body between the right and left eyes is in line with findings of a previous study [19]. This would enable (1) modeling of various unilateral processes that result in a change in local body temperature (e.g., intraocular inflammatory processes) and (2) subsequent assessment of relationships between temperature changes and clinical and anatomical signs of the disease. In addition, the autonomic thermoregulation and stability in the temperature of the ocular surface in the projection of the ciliary body for a small environmental temperature range create good conditions for studies aimed at the development of new methods for quantifying inflammation objectively. Conclusion First, there was no significant difference in the temperature of the ocular surface in the projection of the ciliary body between the right and left eyes in the absence of pathological changes. The mean temperature of the ocular surface in the projection of the ciliary body in intact rabbits was 34.1°С (SD=1.4). Second, temperature of the ocular surface in the projection of the ciliary body in rabbits is characterized by autonomic thermoregulation and is relatively stable at small environmental temperature variations. Finally, the pattern of heat exchange in rabbit’s ocular surface allows for modeling of unilateral ocular pathological processes that would have a change in local body temperature in the projection of the ciliary body as an objective marker.
References 1.Bakbardina LM. [Thermometric diagnostics of inflammatory process in the anterior uvea]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Odesa: Filatov Institute of Eye Disease and Tissue Therapy; 1988. Russian. 2.Eremenko AI. [Thermography in the diagnosis of vascular neuritis of the optic nerve]. Oftalmol Zh. 1990;(4):235-9. Russian. 3.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001 Jul;80(4):263-70. 4.Lahiri BB, Bagavathiappan S, Jayakumar T, et al. Medical applications of infrared thermography: a review. Infrared Phys Technol. 2012 Jul; 55(4): 221–35. 5.Bloch-Michel E Nussenblatt RB. International Uveitis Study Group: recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987 Feb 15;103(2):234-5. 6.Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005 Sep;140(3):509-16. 7.Konstantopoulou K, Del'Omo R, Morley AM, et al. Comparative study between clinical grading of anterior chamber flare and flare reading using the Kowa laser flare meter. Int Ophthalmol. 2015 Oct;35(5):629-33. 8.Mahendradas P, Khanna A, Kawali A, Shetty R. Quantification of inflammation in inflammatory eye diseases. IJRCI. 2014;2:1–4. 9.Kempen JH, Ganesh SK, Sangwan VS. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008 Dec;146(6):813-8.e1. 10.Astakhov IuS, Kuztetsova TI. [Laser flare photometry in clinical practice]. Oftalmologicheskie vedomosti. 2016;9(2):36-44. Russian. 11.Guney E, Tugal-Tutkun I. Symptoms and Signs of Anterior Uveitis. US Ophthalmic Rev. 2013;6(1):33-7. 12.Herbort CP, Guex-Crosier Y, de Ancos E, et al. Use of laser flare photometry to assess and monitor inflammation in uveitis. Ophthalmology. 1997 Jan;104(1):64-71. 13.Ladas JG, Wheeler NC, Morhun PJ, et al. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. 2005 Jan-Feb;50(1):27-47. 14.Tugal-Tutkun I, Herbort CP. Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int Ophthalmol. 2010;30:453–64. 15.Gonzales CA, Ladas JG, Davis JL, Feuer WJ, Holland GN. Relationships Between Laser Flare Photometry Values and Complications of Uveitis. Arch Ophthalmol. 2001 Dec;119(12):1763-9. 16.Yeo TH, Ilangovan S, Keane PA, et al. Discrepancies in assessing anterior chamber activity among uveitis specialists. Jpn J Ophthalmol. 2016 May;60(3):206-11. 17.Chernookova VA. [Clinical and functional patterns of oculo-ocular reflexes in unilateral mechanical ocular trauma]. [Abstract of a Thesis for the Degree of Cand Sc (Med)]. Moscow: Moscow Helmholtz Research Institute for Eye Diseases; 2007. Russian. 18.Chereshnev VA, Gusev EYu, Yurchenko LN. [Systemic Inflammation: Myth or Reality?]. Vestnik rossiiskoi nauki. 2004;74(3):219-27. Russian. 19.Anatychuck LI, Pasyechnikova NV, Zadorozhnyy OS, et al. [Original device and approaches to the study of temperature distribution in various eye segments (experimental study). J. Ophthalmol. (Ukraine). 2015;6:50-3. 20.Schwartz B. Environmental temperature and the ocular temperature gradient. Arch Ophthalmol. 1965 Aug;74:237-43. 21.Anatychuck LI, Pasyechnikova NV, Zadorozhnyy OS, et al. [Temperature distribution in various compartments of the rabbit eye at various environmental temperatures].Oftalmologiia. Vostochnaia Evropa. 2015;4:60-8. Russian. 22.Freeman RD, Fatt I. Environmental influences on ocular temperature. Invest Ophthalmol. 1973 Aug;12(8):596-602.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|