J.ophthalmol.(Ukraine).2020;1:40-48.
|
http://doi.org/10.31288/oftalmolzh202014048 Received: 23 December 2019; Published on-line: 21 February 2020 Treatment of rhegmatogenous retinal detachment: from the past to the future M. Iu. Krongauz, Ophthalmologist; I.O. Nasinnyk, Cand Sc (Med); N.V. Pasyechnikova, Associate Member of the NAMS of Ukraine, Dr Sc (Med), Prof. Filatov Institute of Eye Diseases and Tissue Therapy, National Academy of Medical Science of Ukraine; Odesa (Ukraine) E-mail: mashakrongauz@gmail.com TO CITE THIS ARTICLE: Krongauz MIu, Nasinnyk IO, Pasyechnikova NV. Treatment of rhegmatogenous retinal detachment: from the past to the future. J.ophthalmol.(Ukraine).2020;1:40-48. http://doi.org/10.31288/oftalmolzh202014048
Rhegmatogenous retinal detachment (RRD) is a severe disorder that can potentially lead to blindness. The traction developing in pathological attachment of the vitreous to retinal degeneration areas and in posterior hyaloid detachment leads to formation of retinal breaks and subsequent retinal detachment. The approach to treatment of RRD has changed with years from cautery (J. Gonin, 1920) to modern vitrectomy (R. Machemer, 1973). Current advances in vitrectomy are related mostly to the three major fields: development of advanced vitrectomy tools, novel operative techniques and tamponade agents. The tamponade agents used in vitrectomy have specific advantages and disadvantages. Therefore, new solutions are being sought to minimize harmful effects of tamponade agents on ocular tissues, which would make the process of rehabilitation more comfortable for patients. In recent decades, the potential for using hydrogels as a substitute to the vitreous removed during vitrectomy has been actively explored (Widder, 1960, Balazs, 1972, Malson, 1985). The first commercially available biomedical sodium hyaluronate product for use in eye surgery, Healon, was developed by Balazs. Although Healon was developed as a vitreous substitute, it has been successfully used also in cataract surgery, and truly initiated the age of viscosurgery. The research on the potential for using hydrogels as a visual substitute is underway. Keywords: vitrectomy, silicone oil, gas tamponade, rhegmatogenous retinal detachment, hydrogel, viscosurgery
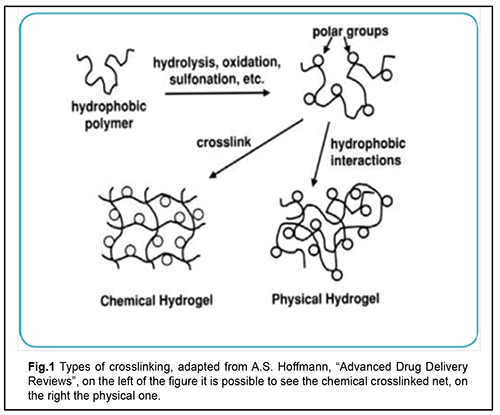
Causes and mechanisms of RRD Rhegmatogenous retinal detachment (RRD) is a severe disorder that can potentially lead to blindness [1-3]. RRD incidence has been reported to be between 6.3 and 17.9 per 100,000 population. For studies with a sample size >300 the mean proportion of bilateral RRD was 7.26% [1, 3]. RRD is more common in men (?60%) than in women [4]. Eighty-four percent of RRD patients are of the working age. The incidence of surgery requiring RRD showed a bimodal distribution across age groups, with one peak representing patients between 65 and 69 years of age and the second peak representing patients between 20 and 29 years of age [3, 4]. RRD is closely related to the detachment of the posterior face of the vitreous body (the posterior hyaloid), which may separate from the internal limiting membrane (ILM) of the retina [5]. The vitreous is composed of 98%-99% water, hyaluronic acid and different types of collagen [6-8]. During the detachment of the posterior hyaloid membrane, vitreous gel exhibits changes in rheological properties, cortical vitreous cells (hyalocytes) reduce in number, and hyaluronic acid (HA) balance becomes impaired, which results in liquefaction and fractionation of the vitreous, leading to a reduced adhesion of the cortical vitreous [9]. Hyaluronic acid has high osmolarity and is able to absorb an amount of water equal to 4,000-fold its dry weight [8, 10]. A reduced concentration of HA in the vitreous results in filling of deficient vitreous volume by low-viscosity fluid and the fluid portion of the cortical vitreous which fill the retro-vitreous space [11]. In pathological attachment of the vitreous to retinal degeneration areas, the traction developing during posterior hyaloid detachment results in eventual formation of retinal breaks [12]. Low-viscosity fluid from the vitreous cavity prolapses into the subretinal space through retinal tears, and separates the sensory retina from the pigment epithelium (RPE) [13]. Forces maintaining retinal attachment include hydrostatic pressure, oncotic pressure, and active transport; they ensure adhesion between the rod and cone cell layers, RPE and choroid. Rhegmatogenous RD develops when the forces promoting retinal detachment overwhelm the forces maintaining retinal attachment [4]. This results in the development of photoreceptor hypoxia and ischemia, apoptosis of macular cone photoreceptors [14], while gravitation and active eye movements contribute to spread of the subretinal fluid (SRF) and extension of retinal detachment [13]. RRD is more common in the age group of 40 to 80 years. In addition, it has been reported that, of the cases with RRD, 0.3% to 30% were associated with RRD of the fellow eye; 40% to 82% with myopia; 30% to 50% with aphakic eyes; ?10% with artiphakic eyes; 20% to 30% with lattice degeneration; and 10% to 20% with direct eye trauma. Moreover, a portion of RRD patients had Marfan’s syndrome or Stickler syndrome, senile retinoschisis or a history of retinal breaks. Methods of treatment for RRD Current methods of treatment for RRD are aimed at removing a major pathogenetic factor (closure of retinal defects through the formation of chorioretinal adhesions [15]). In 1920, Gonin reported the first successful reattachment of the retina by sealing the retinal break to the underlying RPE and the choroid using a cautery. This procedure was followed by the formation of scar tissue at the site of cautery [16]. Complications occurred following the procedure, which made researchers to search for new methods and tactics of treatment for RRD. Diathermy was introduced by Weve and Safar in 1931It was used either on the bare sclera (surface diathermy) or after trephining the sclera (penetrating diathermy). Drainage of SRF was performed along with diathermy. The retinal reattachment rate after the procedure was as high as 70% [17]. The diathermy by Lindner and Safar [15, 17] required neither determining the size of nor localizing the leaking break since the intent was to create a “barrier” of retinal adhesions posterior to the leaking break. As a result, the treatment was no longer limited to the break, but was expanded over the quadrant in which the break or presumed breaks were located. The break itself, however, remained open, and started to leak again to the subretinal space, resulting in a redetachment [15]. Further research was aimed at reinforcing the barrier. For this purpose, it was decided to bring the retina and choroid closer to the retina through segmental buckling with different materials. This approach was used to form an indentation wall that along with diathermy had to prevent the extension of the detachment. The break at the front edge of the buckle could, however, leak again to the subretinal space, resulting in a redetachment which subsequently crossed the barrier of coagulations [15, 17]. The former segmental buckle barrier was expanded to a circular plombe in 1953 by Schepens. The cerclage operation with drainage of subretinal fluid evolved. Silk suture was used to encircle the equator of the eye and to create a new ora serrata encircling both visible and latent defects; this, however, could not prevent a redetachment, since the break, positioned at the anterior edge of the buckle, was not sufficiently tamponaded [15]. Subsequently, the cerclage technique was refined, and the rate of reattachment further increased to 78-80% [15,17]. According to the 15-year follow-up data of the study by Kreissig and Simander, the reattachment rate after segmentary buckling was not higher than after circumferential buckling [15]. In addition, the cerclage technique may result in complications associated with eye compression (posterior pole ischemia, elevated intraocular pressure (IOP), degenerative scleral changes, and increased axial length of the globe [15,18]). Moreover, SRF drainage has resulted in complications like vitreous hemorrhage (15.8%), choroidal detachment (8%), and trapped vitreous or retina [15, 17]. Further evolution of the cerclage technique included the development of more tissue-inert materials for the plombe itself and novel methods for creating strong adhesion. Thus, the modified Custodis procedure was developed, which included cryopexy and local scleral identation with an inert silicone plombe, resulting in reattachment rates as high as 79.8-92.6% [15, 19]. In 1938, Rosengren reported the first large series of patients treated with intraocular air injection, drainage of SRF, and diathermy. He used an intraocular air bubble to tamponade ab interno a break after scleral diathermy and drainage of SRF. However, the air absorbed as soon as day 2 or 3 after surgery, and the time was too short to form the chorioretinal adhesion [15, 18]. In 1967 Lincoff refined the gas tamponade technique by changing air to sulfur hexafluoride with an expansion of 1.9x. Hilton et al. re-introduced the gas technique without drainage (pneumatic retinopexy) in 1986. In pneumatic retinopexy, a gas bubble is injected into the vitreous cavity for the surgical repair of non complicated retinal detachments [15]. Perfluoroethane (CF3CF3) has a long half-life of 10-12 days and has been used for this methodology since 1980 [15,20]. The method, however, also resulted in complications like proliferative vitreoretinopathy (PVR) in 4% of cases, new retinal breaks in 15% of cases, and retinal re-detachment in 23-38% [20]. In 1971, Machemer described a new method of treatment for RRD, vitrectomy. Vitrectomy was employed by Machemer for retinal detachments complicated by vitreous traction and vitreoretinal proliferation [21]. By applying vitrectomy as a primary procedure for RRD, traction on the leaking breaks or tears and as well the anterior and posterior vitreous was removed. Vitrectomy involves removal of pathologically changed vitreous, smoothing out the retina, and sealing retinal breaks with intravitreal tamponade with silicone oil or a mixture of air and gas [22]. Various studies reported a success rate for vitrectomy of 69.7% to 94%. Arya et al [23] reported a meta-analysis of published studies from 1966 to 2004 regarding surgical treatment of pseudophakic retinal detachments. Two thousand two hundred thirty eyes participated: 1579 operated by SB, 457 by PPV, and 194 by the combined method of PPV and SB. The meta-analysis implied that PPV with or without SB was more likely to achieve a favorable anatomical and visual outcome than conventional SB alone in uncomplicated pseudophakic RDs. Ideal tamponade substances: a search has been going on for more than 100 years. The search for the best substitute for the removed vitreous has been going on for more than 100 years. Knapp was first to introduce sodium chloride (0.75%) as vitreous substitute in cataract extraction surgery in 1900. Physiologic saline (0.9%) became the most common vitreous substitute due to its availability; however, it has a low ability to act as a tamponade agent because it has a low viscosity and its resoption takes place within a day after surgery (Winder 1962) [24]. One of the earliest descriptions of vitreous substitutes dates to 1911 when Ohm treated retinal detachment with injection of sterile air into the vitreous cavity and drained subretinal fluid through a retinotomy [25]. Nevertheless, it was not until 1935, after the acceptance of Gonin's principles of closing retinal breaks that surface diathermy was supplemented with air injection. In 1938, Rosengren stressed that facedown positioning is required to tamponade retinal breaks. In 1966, Ivaschenko demonstrated that resorption of the intravitreal air bubble takes place within 72 hours to 144 hours [2, 24]. The use of sulphur hexa?uoride (SF6), the gas that is five times heavier than air, as a tamponade agent, was devised by Norton. SF6 is an inert and non-toxic gas that expands intraocularly 1.9 times its original volume, and persists in the eye for seven days [26]. Perfluorocarbon gases (CF4; C2F6; C3F8; and etc.) have been introduced as tamponade agents by Lincoff in early eighties. They are inert and non-toxic gas that 1.9 to 5 times their original volumes and the half-life of these gases range between 6 and 45 days [27]. The use of silicone oil, specifically, polydimethylpolysiloxane, as a long-acting tamponade agent was pioneered by Cibis in 1962. Due to the high viscosity and surface tension of the gas bubble silicone oil, an effective seal of the retinal breaks at most locations can be achieved. However, even modern high-purity silicone oils contain small amounts of impurities (unpolymerized/unconverted monomers, low-molecular-weight cyclosiloxanes, chemically reactive hydroxyl end groups, ions of heavy metals, and residual catalyzers) that may cause various complications. Keratopathy develops whenever silicone oil gets into the anterior chamber. The first use of liquid perfluorocarbon (PFCL) as a tamponade agent was reported by Haidt and co-workers in 1982. That study, however, showed that the PFCL caused irreversible changes in all ocular tissues, possibly on account of mechanical pressure on the retina [28]. In addition, since the late twentieth century, there have been reports on experimental vitreous replacement with highly viscous and high surface tension PFCL (e.g., perfluoropolyether DK-164) for short-term vitreous tamponade. PFCL are heavier than water, and, therefore, contribute to the apposition of the retina to the underlying tissue, facilitating the formation of firm chorioretinal adhesions for effective tamponade of retinal breaks. Similar to their predecessors, modern PFCL are, however, biologically active compounds. They form the film on the surface of the retina; the film firmly adheres to the retina, and is extremely difficult to destroy mechanically or to remove completely from the eye [29,30]. Gao et al [31,32] devised a novel capsular artificial vitreous using tailor-made silicone rubber elastomer. The novel artificial vitreous body consists of a thin vitreous-like capsule with a silicone tube-valve system. The capsule can be folded and implanted into vitreous cavity through 1.5 mm incision on sclera. Physiological balanced solution (PBS) was then injected into the capsule and inflated to support retina and control intraocular pressure (IOP) through the tube-valve system subsequently fixed under the conjunctiva. Experiments using rabbits showed that the novel vitreous body could effectively support the retina and apparently induced no significant pathological changes in the eye over 8 weeks. In the late nineteenth century, Weber was the first to propose to replace vitreous with homologous or heterologous vitreous in an attempt to treat patients with retinal detachment [33]. In the early twentieth century, the idea was further developed by Deutschmann (1895,1906,1926) who carried out heterotransplantations with rabbit vitreous and calf vitreous. Later on, in a study by Cutler (1946), in all transplants, the donor vitreous was drawn out through a needle and injected into the recipient posterior chamber, rather than being removed and transplanted as a whole. The author concluded that the human donor vitreous drawn out through a needle and injected into the recipient posterior chamber was tolerated by the recipient better than the heterologous donor vitreous [31, 33, 34]. In 1976, Shafer reported on a case series of 200 human vitreous transplants performed for retinal detachment. The vitreous was planted using an 18-gauge needle through a pars plana incision with the needle tip just posterior to the recipient lens. The most frequent post-operative complication was mild vitreous haze that disappeared without treatment after 2--5 days. Uveitis occurred in 3.5% of cases. The ?rst documented animal experiment using a rabbit model with control groups was performed in 1947 by Katzin and Blum [31]. Twenty-four rabbit eyes had native vitreous removed and rabbit donor vitreous injected with a follow-up period of six months. The most common complication was vitreous haze (20 of 24 animals) that persisted at six months in two animals. Replacement of vitreous with spinal fluid was first done by Hegner in 1928. In the mid-twentieth century, Stukalov demonstrated that spinal fluid has some advantages over autologous, homologous, or heterologous donor vitreous and physiological saline, but its clinical use as a vitreous substitute has been limited by its low viscosity and difficulties associated with its harvesting and storage [24,33]. In 1958 Balazs suggested that hyaluronic acid (HA) preparations be used for replacement of the vitreous body of the eye [35,36,37]. Widder (1960) and Hruby (1961) used commercially available HA of bovine origin for vitreous replacement in human eyes after animal studies. According to Widder, the residence time for 80% of HA implanted into the vitreous cavity was 8 days [24,33]. In subsequent 10 years, Hruby (1959, 1961, 1963), Castren (1964), Balazs (1972), Bordiugova (1966) reported on subsequent experimental studies with hyaluronic acid vitreous substitutes. Lauronite, a hyaluronic acid vitreous substitute (0.2% hyaluronic acid) was developed by Bordiugova et al in 1960. It was injected endovitreally in animals after experimental post-traumatic loss of their natural vitreous. Lauronite, however, was not tested in clinical studies due to failure to remove a large amount of protein fractions that made the substance non-transparent [24,37]. Hyaluronic acid/1% sodium hyaluronate (Healon) was developed in 1977, and, when injected into the vitreous cavity, remained there for up to 14 days; it, however, failed to provide for effective long-term tamponade [37, 38]. In a rabbit study by Malson and Lindqvist (1985), vitrectomy was followed by implantation of either hyaluronic acid hydrogel (HAH) at various concentrations (fine HAH containing 0.1% to 2.5% of solid matter by weight) or gel globules (10% to 50% of solid matter by weight) through a 6-mm incision [39]. The HAH, however, was not tested in clinical studies, because HAH swelling was poorly controlled due to the hydrophilic nature of HAH, and it was difficult to use the HAH [40]. Disadvantages of tamponade agents Modern tamponade agents have a number of disadvantages. Intravitreal silicone oil tamponade may result in perisilicone proliferation, cataract progression, silicone oil emulsification, and requirement for re-surgery for silicone oil removal [41]. In addition, penetration by silicone particles of the anterior chamber angle impairs aqueous outflow, leading to secondary ophthalmic hypertension [42] and secondary glaucoma (4% to 40% of cases) [19,64]. When injected into the eye for tamponade, silicone oil gets into almost all ocular coats, and induces chronic inflammation in them, which results in significant degenerative changes [44]. A disadvantage of air or gas tamponade for RRD is a requirement for long-term face-down positioning postoperatively. This results in difficulties in patients with general medical conditions; absence of pattern vision in intraocular gas bubbles with volumes exceeding 45%; early cataract formation; and difficulties in sealing of inferior retinal breaks and performing laser coagulation early after surgery [45]. Temporary need to avoid higher altitudes and air travels is another disadvantage of gas tamponade [46]. Moreover, after vitrectomy, there is a risk of developing primary open-angle glaucoma [47]. Current advances in vitrectomy are related mostly to the three major fields: development of advanced vitrectomy tools, operative techniques and tamponade agents [8, 18, 48, 49]. Hydrogels: definition Hydrogel is a colloid gel in which the liquid component is water. Hydrogels are three-dimensional polymers that swell in aqueous solutions without dissolving, and were the ?rst biomaterials synthesized for human use [50,51]. They swell in aqueous solutions without dissolving, and thus produce a semisolid and jelly-like material with a certain (usually low) strain of plastic flow and the properties between those of solution and fine suspension. Water absorption and hydrogel kinetics may be controlled by selecting the chemical structure, cross-linking and foaming. Depending on target applications, absorption of water by hydrogels may range from as small as tenths of a percent to as large as that related to several hundred fold increase in volume [49]. Hydrogel classification Hydrogels can be classified based on their origin as natural polymers (and their derivatives), synthetic polymers and composite (natural plus synthetic). Natural polymers can be divided in anionic (e.g., hyaluronic acid, pectin, and chondroitin sulfate), cationic (chitosan), amphipathic (collagen, chitin and fibrin) and neutral (dextran and agarose). Synthetic polymers are a diverse group, including, e.g., polyesters such as poly(ethylene glycol)–polylactide–poly(ethylene glycol) (PEG–PLA–PEG) or poly(N-isopropylacrylamide-co-acrylic acid) [p(NIPAAm-co-AAc)] with cross-linking agents. Composite polymers are combinations of natural and syntheric polymers (e.g., collagen-acrylate and alginate-acrylate) [49]. Hydrogels may be chemically stable or they may degrade eventually disintegrate and dissolve; the latter are called reversible or physical hydrogels. In physical hydrogels, the network is held together by molecular entanglements and/or forces like ionic, H-bonding, or hydrophobic forces. Primary hydrogels are non-reversible, or chemical hydrogels with their networks held together by covalent bonds. Similar to physical hydrogels, chemical hydrogels are not homogenous [52]. Since late last century, the gels used in eye surgery have been conventionally called viscoelastics. In 1979, Balazs coined the term 'viscosurgery' for surgeries involving the use of viscoelastic solutions. Viscosurgery uses these agents to protect cells from mechanical trauma, to maintain or create tissue spaces, and to ensure separation and lubrication of tissue surfaces. Since the first decade of this century, the term “Ophthalmic Viscoelastic Device (OVD)” has been used in the literature, stressing a wide range of viscoelastic functions in surgery. Viscoelastics are divided into disperse (or adhesive) and cohesive, with the former having lower viscosity than the latter. In addition, the former and the latter have a molecular weight below and above 1000,000 daltons, respectively [35]. Hyaluronic acid HA is found all over the body in various concentrations depending on the type of tissue, which stresses the important biological role of HA and points to its potential for use in clinical practice [55]. It transmits a variety of mechanical and biological signals to surrounding cells and tissues [56], exerting an effect on cell adhesion and neural migration and propagation [57]. In vivo models have been developed to determine the biophysical features of HA required for these purposes [55, 58]. Hyaluronan receptors (CD44, CD168, and etc) enable the body to control HA concentration in the extracellular matrix and in the cells through HA synthesis, organization and degradation [57,59,60] in the presence of hyaluronidase [8,10]. HA is widely used in medicine as a lubricant for intraarticular injection in osteoarthritis treatments and as a feeler in facial contouring [55]. The first biomedical sodium hyaluronate product, Healon, was developed for use in eye surgery in the nineteen seventies, as mentioned above [37, 38]. In the initially developed hydrogels, swelling was poorly controlled [31, 39, 59], and hydrogel turbidity increased due to accumulation of protein structures at some time after injection [24]. In addition, animal studies [61, 62] found some of them (Bio-Alcamid®, a nonreabsorbable polyacrylamide hydrogel composed of polyalkyl-imide-groups, and polyvinylpyrrolidinone (PVP)) to be toxic for eyes. Dozens of hyaluronic acid viscoelastics are available for ophthalmic applications; they are used to maintain anterior chamber volume and for corneal endothelial protection in cataract surgery and as drainage implants in glaucoma [54, 63]. Hyaluronic acid hydrogels have been considered for use as intravitreal systems for controlled release of medical agents [63, 64] or mesenchymal stem cells [65]. In addition, there have been numerous experimental studies aimed at the development of methodologies for using hydrogels as vitreous substitutes. Experimental studies have been conducted to investigate not only hydrogel components, but also methods for hydrogel stabilization [31, 62, 66, 67]. Some of these hydrogel types have been already tested as vitreous substitutes in in vivo and in vitro studies [32, 34, 68, 69]. Su and colleagues [66] investigated in vitro an injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel (oxi-HA/ADH) as a vitreous substitute. The refractive index of this hydrogel ranged between 1.3420 and 1.3442. In addition, the hydrogel showed no significant pathological biological response from the RPE or cornea within the 3-month follow-up. Barth and co-authors [61,68,70] studied biological effects of a cross-linked hyaluronic acid hydrogel (Healaflow®) on retinal explants of adult rats. They demonstrated that Healaflow exerts no effect on retinal morphology, and noted somewhat increased regulation of glial fibrillar acidic protein (GFAP) and activation of Muller cells [61,68,70]. Schichels and colleagues [69] evaluated the efficacy of two novel artificial vitreous body substitutes (VBS) consisting of highly biocompatible thiolated cross-linked hyaluronic acid (HA)-based hydrogels in a model of retinal detachment in 24 rabbits. Formulations were prepared in physiological phosphate buffer and contained either 2.2% or 1% HA. At the end of the surgical procedure the respective hydrogel (VBS strong or VBS soft) was injected into the vitreous cavity until egress of the tamponading agent through the opposite sclerotomy was noted. Two different hydrogel formulations, VBS strong and VBS soft, were prepared from thiolated HA by induction of disulfide bridge formation, and provided for the osmotic pressure required for retinal re-attachment. In the hydrogel treated eyes, the retina stayed attached in the majority of the cases (73.3%). IOP and retinal morphology were normal as long as the retina remained re-attached. Januschowski and co-authors (2019) [71] evaluated biophysical properties of a 1% thiolated HA-based hydrogel. Specifically, they assessed the effect exerted by the hydrogel on IOP (in enucleated porcine eyes) and toxicity (in isolated bovine retinal explants). Although the results obtained in an ex vivo model can be considered satisfactory, the hydrogel exposure time did not exceed 24 hours, which warranted for in vivo studies for further testing of the developed hydrogel [71]. Conclusion HA hydrogels have a potential to become a new group of tamponade agents for use in vitreoretinal surgery. They are highly biocompatible and, due to their three-dimensional structure and controllable swelling, can exert the required pressure on the retina until firm chorioretinal adhesions form [62, 72]. The following aspects, are, however, still poorly understood: light-, pressure-, and electric field-dependent activity and reactivity of HA hydrogels; and interaction of HA hydrogels with ocular tissues, medical agents than may be introduced into the vitreal cavity, laser radiation, and other chemical substances or physical stimuli [73, 74].
References 1.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010 Jun 9; 94(6):678–6. DOI: 10.1136/bjo.2009.157727. 2.Zakharov VD. [Retinal detachment surgery]. [Abstract of Dr Sc (Med) Dissertation]. Moscow: MNTK Eye Microsurgery; 1985. Russian. 3.Osmanov RE. [Prevalence of rhegmatogenous retinal detachment in Tambov region]. Vestnik TGU. 2015;20(6):1666-8. Russian. URL: https://cyberleninka.ru/article/v/rasprostranennost-regmatogennoy-otsloy.... 4.Park SJ, Choi NK, Park KH, Woo SJ. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One. 2013 Nov 13; 8(11): e80174. DOI: 10.1371/journal.pone.0080174. 5.Sebag J. Anatomy and pathology of the vitreo-retinal interface. Eye. 1992 Nov 1; 6, 541–552 DOI:10.1038/eye.1992.119. 6.Simone D, Caprani SM, Airaghi G, Vinciguerra R, Bartalena L, Testa F, Mariotti C, Porta G, Simonelli F, Azzolini C. Vitreous Substitutes: The Present and the Future. BioMed Research International. 2014 May 4; 2014, 12. DOI: 10.1155/2014/351804. 7.Vit VV. [The structure of the human visual system]. Odessa: Astroprint; 2010. Russian. 8.Balazs EA, Laurent TС, Laurent UBG. Studies on the structure of the vitreous body. VI. Biochemical changes during development. J Biol. Chem. 1959 Feb; 234(2):422–8. 9.Sebag J. The Vitreous — Structure, Function, and Pathology. N.Y.: Springer; 1989. 10.Hench L, Jones J. Biomaterials, Artificial Organs and Tissue Engineering. 1 ed. London: Woodhead Publishing; 2005. 11.Boiko EV, Maltsev DS, Suetov AA. [Posterior hyaloid detachment: concept, prevalence, classifications, and possible causes]. Oftalmologicheskiie vedomosti. 2009; 2(3):39–47. Russian. https://cyberleninka.ru/article/n/otsloyka-zadney-gialoidnoy-membrany-po.... 12.Zakharov VD. [Vitreoretinal surgery]. Moscow: Moscow; 2003. Russian. https://www.twirpx.com/file/519398/. 13.Lin Т, Mieler WF. Management of primary rhegmatogenous RD [Internet]. Chicago: Rev. Ophthalmol. Online; 2008 July 15. Available from: http://www.reviewofophthalmology.com/content/d/retinal_insider/i/1226/c/.... 14.Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005 Apr.; l39(4): 605 5. DOI:10.1016/j.ajo.2004.11.046. 15.Galimova AB. [Evolution of approaches to management of rhegmatogenous retinal detachment]. Oftalmologicheskiie vedomosti. 2011; 4(3):70–7. Russian. URL: https://cyberleninka.ru/article/v/evolyutsiya-podhodov-k-hirurgicheskomu.... 16.Rumiantseva AF. [Eye surgery]. Kyiv: Gostmedizdat USSR; 1957. Russian. 17.Kreissig I. [Development of Retinal Detachment Surgery: How it came about and what we are doing now (part I)]. Russkii meditsinskii zhurnal. 2007; 8(4):163–7. Russian. 18.Kreissig I. A Practical Guide to Minimal Surgery for Retinal Detachment, Volume 1: Diagnostics, Segmental Buckling without Drainage, Case Presentations. 1st ed. New York: Thieme; 2000. 19.Ahmadieh H, Moradian S. et al. Anatomic and visual outcomes of sclera buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachments. Ophthalmology. 2005 Aug;112(8):1421–4. DOI:10.1016/j.ophtha.2005.02.018. 20.Lincoff H, Coleman J, Kreissig I, et al. Theperfluorocarbongases in the treatment of retinal detachment. Ophthalmology. 1983 May;90(5):546–5. DOI:10.1016/s0161-6420(83)34525-5. 21.Machemer R. Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971 May;75(4):813–7. PMID:4667660. 22.Schneider E, Schneider E, Geraets R, Johnson M. Pars plana vitrectomy without adjuvant procedures for repair of primary rhegmatogenous retinal detachment. Retina. 2012 Feb; 32(2):213–6. DOI:10.1097/IAE.0b013e3182278b29. 23.Arya AV, Emerson JW, Engelbert M, Hagedorn C, Adelman RA. Surgical management of pseudophakic retinal detachment: A meta-analysis. Ophthalmology. 2006 Oct;113(10):1724–8. DOI:10.1016/j.ophtha.2006.05.044. 24.Maliatsinskii IA. [Clinical and functional substantiation of the technique for microinvasive surgical treatment of recurrent inferior retinal detachment during intravitreal silicone oil tamponade]. [Abstract of Cand Sc (Med) Thesis]. Moscow: MNTK Eye Microsurgery; 2015. Russian. URL: http://www.mntk.ru/files/upload/avtoreferat-malyatsinskiy.pdf. 25.Rozhko et al. [Efficacy of extrascleral surgery with pneumatic retinopexy with sulfur hexafluoride (SF6) for rhegmatogenous retinal detachment]. Rossiiskaia oftalmologiia on-line. 2009. URL: article.aspx. 26.Norton EWD, Aeberg T, Fung W, Curtin VT. Giant retinal tears. I. Clinical management with intravitreal air. Am J Ophthalmol. 1969; 68(6):1011-10. 27.Lincoff A, Kriessig I. Intravitreal behavior of perfluorocarbons. Dev Ophthalmol. 1981; 2: 17-6. DOI:10.1159/000395297. 28.Sandner D, Engelmann K. First experiences with high-density silicone oil (Densiron) as an intraocular tamponade in complex retinal detachment. Graefe's Arc Clin Exp Ophthalmol. 2006 May; 244(5): 609-10. DOI:10.1007/s00417-005-0110-8. 29.Zhmurik DV. [Effect of 30 day tamponade using perfluorocarbon liquid on the bioelectric functional activity and structure of the retina in rabbits]. J Ophthalmol (Ukraine). 2016; 1(468): 58-7. Russian. http://nbuv.gov.ua/UJRN/Ofzh_2016_1_15. 30.Lyskin PV, Kazimirova EG, Perepukhov AM. [Physical and chemical aspects of double intravitreal tamponade with PFCL and SO]. Oftalmokhirurgiia. 2014; 1: 64-7. Russian. URL: https://doi.org/10.25276/0235-4160-2014-1-64-67. 31.Kleinberg TT, Tzekov RT, Stein L, Ravi N, Kaushal S. Vitreous substitutes: a comprehensive review. Survey of Ophthalmology. 2011 May 24;56(4):300-23. DOI:10.1016/j.survophthal.2010.09.001. 32.Gao Q, Mou S, Ge J, et al. A new strategy to replace the nanural vitreous by a novel capsular artificial vitreous body with pressure-control valve. Eye. 2008 Apr;22(3):461-8. DOI: 10.1038/sj.eye.6702875. 33.Leparskaia NL. [Role of proliferative vitreoretinopathy in the pathogenesis, clinical picture and treatment of traumatic retinal detachment (clinical and experimental study)]. [Abstract of Cand Sc (Med) Thesis]. Moscow: MNTK Eye Microsurgery; 2005. Russian. URL: https://www.dissercat.com/content/eksperimentalnoe-obosnovanie-fotodinam.... 34.Cutler N.L. Transplantation of human vitreous: a preliminary report. Arch Ophthal. 1946 June; 35(6):615-8. DOI: 10.1001/archopht.1946.00890200630002. 35.Petrov SIu, Mazurova IuV, Aslamazova AE, et al. [Use of viscoelastics in eye surgery]. Natsionalnyi zhurnal glaucoma. 2016; 15(1): 97-104. Russian. 36.Balazs EA. Physical chemistry of hyaluronic acid. Fed Proc. 1958 Dec; 17(4):1086-7. PMID:13619778. 37.Balazs EA, Freeman MI, Kloti R, Meyer-Schwickerath G, Regnault F, Sweeney DB. Hyaluronic acid and replacement of vitreous and aqueous humor. Mod Probl Ophthalmol. 1972; 10(3):21. PMID:4626802. 38.Miller D, Stegmann R. Healon. A guide to its use in ophthalmic surgery. New York: John Wiley; 1983. 39.Malson T, Lindqvist BL, inventors; PHARMACIA AB., assignee. Gel of crosslinked hyaluronic acid for use as a vitreous humor substitute. United States patent US 4716154A. 1984 Jan 08. 40.Suri S, Banerjee R. In vitro evaluation of in situ gels as short term vitreous substitutes. J Biomed Mater Res A. 2006 Dec 1;79(3):650–14. DOI:10.1002/jbm.a.30917. 41.Inoue M, Iriyama A, Kadonosono K, Tamaki Y, Yanagi Y. Effects of perfluorocarbon liquids and silicone oil on human retinal pigment epithelial cells and retinal ganglion cells. Retina. 2009 May;29(5):677-4. 42.Ichhpujani P, Jindal A, Katz J. Silicone oil induced glaucoma: A review. Graefes Arch Clin Exp Ophthalmol. 2009 Dec;247(12):1585-8. DOI: 10.1007/s00417-009-1155-x. 43.Ahmadieh H, Moradian S. et al. Anatomic and visual outcomes of sclera buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachments: six-month follow-up results of a single operation--report no. 1. Ophthalmology. 2005 Aug;112(8):1421-8. 44.Soloviova EP. [Silicone distribution in ocular tissue after vitrectomy with replacement using silicone oil]. Oftalmologicheskiie vedomosti. 2012; 5(1):18–21. Russian. 45.Sigler EJ, Randolph JC, Charles S, Calzada JI. Intravitreal fluorinated gas preference and occurrence of rare ischemic postoperative complications after pars plana vitrectomy: a survey of the American Society of Retina Specialists. J Ophthalmol. 2012 Sep 11; 2012:230596-5. 46.Lincoff H, Weinberger D, Stergiu P. Air travel with intraocular gas. II. Clinical considerations. Arch Ophthalmol. 1989 Jun;107(6):907-3. 47.Malucelli G, Malucelli G, Dore J, Sanna D, Nuvoli D, Rassu M, Mariani A, Alzari V. Sliding Cross-linked Thermoresponsive Materials: Polypseudorotaxanes Made of Poly(N-Isopropylacrylamide) and Acrylamide-?-Cyclodextrin. Fronti Chem. 2018 Nov 23;6:585. DOI: 10.3389/fchem.2018.00585. 48.Sara S, Hutter V, Brown MB, Cook MT, DYS Chau. Diffusion through the ex vivo vitreal body - Bovine, porcine, and ovine models are poor surrogates for the human vitreous. Int J Pharm. 2018 Oct 25;550(1-2):207-8. 49.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002 Jan 17;54(1):3-9. DOI:10.1016/s0169-409x(01)00239-3. 50.Wichterle O, L?m D. Hydrophilic gels for biological use. Nature. 1960 Jan 09;185:117-1. https://doi.org/10.1038/185117a0. 51.Kope?ek J, Yang J. Hydrogels as smart materials. Polym Int. 2007 Sep; 56(9):1078 –20. DOI: 10.1002/pi.2253. 52.Khmelnitskii SI, Lesovoi DE. [Prospects for the use of superporous polyvinyl alcohol-based hydrogels and their composites in novel medical technologies]. Novosti meditsiny i farmatsii. 2008; (3):234. Russian. 53.Yahia LH, Chirani N, Gritsch L, et al. History and Applications of Hydrogels. J Biomedical Sci. 2015 Dec; 4:1-23. DOI:10.4172/2254-609X.100013. 54.Petrov SIu, Mazurova IuV, Aslamazova AE, et al. [Use of viscoelastics in eye surgery]. Natsionalnyi zhurnal glaucoma. 2016; 15(1): 97-104. Russian. URL: https://www.glaucomajournal.ru/jour/article/viewFile/164/163. 55.Wolf KJ, Kumar S. Hyaluronic Acid: Incorporating the Bio into the Material [Internet]. ACS Biomater Sci Eng; 2019 Jan 27. Available from: https://pubs.acs.org/doi/full/10.1021/acsbiomaterials.8b01268. 56.Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014 Apr; 10(4):1558–12. DOI:10.1016/j.actbio.2013.12.019. 57.Stern R. Hyaluronan Catabolism: A New Metabolic Pathway. Eur J Cell Biol. 2004 Aug;83(7):317–8. 58.Ponta H, Sherman L, Herrlich PA. CD44: From Adhesion Molecules to Signalling Regulators. Nat Rev Mol Cell Biol. 2003 Jan;4(1):33–12. DOI:10.1038/nrm1004. 59.Baino F. Towards an ideal biomaterial for vitreous replacement: historical overview and future trends. Acta Biomaterialia. 2011 Mar;7(3):921–14. 60.Liang C, Peyman GA, Serracarbassa P, Calixto N, Chow AA, Rao P. An evaluation of methylated collagen as a substitute for vitreous and aqueous humor. Int Ophthalmol. 1998;22(1):13–5. DOI:10.1023/a:1006016809070. 61.Barth H, Crafoord S, O'Shea TM, Pritchard CD, Langer R, Ghosh F. A new model for in vitro testing of vitreous substitute candidates. Graefes Arch Clin Exp Ophthalmol. 2014 Oct; 252(10):1581–11. DOI:10.1007/s00417-014-2714-3. 62.Kope?ek J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28(34):5185–7. DOI:10.1016/j.biomaterials.2007.07.044. 63.Yu Y, Lin X, Wang Q, He M, Chau Y. Long-term therapeutic effect in nonhuman primate eye from a single injection of anti-VEGF controlled release hydrogel. Bioeng Transl Med. 2019 May; 4(2):e10128. DOI: 10.1002/btm2.10128. 64.Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res. 2016 Sep; 41(9): 1216-6. DOI:10.3109/02713683.2015.1101140. 65.Ding SSL, Subbiah SK, Khan MSA, Farhana A, Mok PL. Empowering Mesenchymal Stem Cells for Ocular Degenerative Disorders. Int J Mol Sci. 2019 Apr;20(7):1784. 66.Su WY, Chen KH, Chen YC, Lee YH, Tseng CL, Lin FH. An injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel as a vitreous substitute. J Biomater Sci, Polym Ed. 2011;22(13):1777-20. DOI: 10.1016/j.actbio.2016.07.051. 67.Santhanam S, Lianga J, Struckhoffac J, Hamilton PD, Ravi N. Biomimetic hydrogel with tunable mechanical properties for vitreous substitutes. Acta Biomater. 2016 Oct 1;43: 327-10. DOI:10.1016/j.actbio.2016.07.051. 68.Barth H, Crafoord S, Andr?asson S, Ghosh F. A cross-linked hyaluronic acid hydrogel (Healaflow(®)) as a novel vitreous substitutе. Graefes Arch Clin Exp Ophthalmol. 2016 Apr; 254(4):697–6. DOI:10.1007/s00417-015-3256-z. 69.Schnichels S, Schneider N, Hohenadl C, Hurst J, Schatz A, Januschowski K, Spitzer MS. Efficacy of two different thiol-modified crosslinked hyaluronate formulations as vitreous replacement compared to silicone oil in a model of retinal detachment. PLoS One. 2017 Mar 1;12(3)e0172895 70.Yu AC, Lee YJL, Eng LF. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res.1993 Feb 15;34(3):295—8. DOI:10.1002/jnr.490340306. 71.Januschowski K, Schnichels S, Hurst J, Hohenadl C, Reither C, Rickmann A, Pohl L, Bartz-Schmidt K, Spitzer MS. Ex vivo biophysical characterization of a hydrogel-based artificial vitreous substitute. PLoS One. 2019 Jan 7;14(1):e0209217 DOI:10.1371/journal.pone.0209217. 72.Schramm C, Spitzer S, Henke-Fahle S et al. The crosslinked biopolymer hyaluronic acid as an artificial vitreous substitute. Invest Ophthalmol Vis Sci. 2011 Dec; 53(2):613–8. 73.Gao Tsjan'in, inventor; GUANChZhOU VISBOR BIOTEKNOLODZhI LTD, Assignee. [A method of making collapsible artificial glass body and mould for this end]. Russian Federation Patent RU 2496641C2. Application: 2011118920/05, 30.03.2009. Date of publication: 27.10.2013, Bull. 30. https://patentimages.storage.googleapis.com/b1/f0/de/d355ec127361fa/RU24.... 74.Koo H, Jin G-W, Kang H, et al. A new biodegradable crosslinked polyethylene oxide sulfide (PEOS) hydrogel for controlled drug release. Int J Pharm. 2009 Jun 5;374(1-2):58–7. DOI:10.1016/j.ijpharm.2009.03.010.
|