J.ophthalmol.(Ukraine).2020;1:54-56.
|
http://doi.org/10.31288/oftalmolzh202015456 Received: 21 November 2019; Published on-line: 21 February 2020 A case of vascularized corneal opacity treated with aflibercept A.S. Cholak, MD; I.O. Nasinnyk, Cand Sc (Med); P.O. Kostenko, Cand Sc (Med); S.A. Iakimenko, Dr Sc (Med), Prof.; A.R. Korol, Dr Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy, National Academy of Medical Science; Odesa (Ukraine) E-mail: anastasiakrivoruchko2593@gmail.com TO CITE THIS ARTICLE: Cholak AS, Nasinnyk IO, Kostenko PO, Iakimenko SA, Korol AR. A case of vascularized corneal opacity treated with aflibercept. J.ophthalmol.(Ukraine).2020;1:54-56. http://doi.org/10.31288/oftalmolzh202015456
Background: Corneal neovascularization (CNV) is a serious condition that can lead to a profound decline in vision. Current research on treating CNV with angiogenesis inhibitors is underway in numerous centers, and a number of studies have demonstrated the efficacy of this approach. Purpose: To present a case of corneal neovascularization treated with aflibercept. Material and Methods: An ocular examination included visual acuity assessment, biomicroscopy, anterior eye photography and fluorescein angiography (FA). In addition, an area of CNV was assessed before and 6 months after a single subconjunctival injection of 4 mg (0.1ml) aflibercept. Results: An area of CNV decreased from 38,081 pixels before injection to 20,782 at 6 months, with a reduction in 17,299 pixels. Conclusion: In the case reported here, a single subconjunctival injection of 4 mg (0.1ml) aflibercept was found to be effective for reducing corneal neovascularization at 6 weeks. Keywords: corneal neovascularization, fluorescein angiography, angiogenesis inhibitor, aflibercept
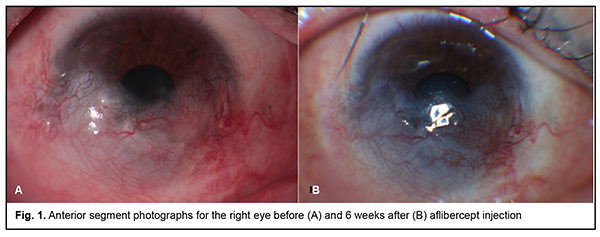
Introduction Corneal neovascularization (CNV) is a serious condition that can lead to a profound decline in vision [1,2]. The abnormal vessels block light, compromise visual acuity, and may lead to aseptic corneal inflammation, scarring and edema [1, 3, 4]. Corneal neovascularization occurs whenever the balance between angiogenic and antiangiogenic factors is shifted toward the former [5, 6]. In addition, CNV is preceded by activation of vascular endothelial growth factor (VEGF), one of the most important mediators of angiogenesis [5]. Current research on treating CNV with angiogenesis inhibitors is underway in numerous centers, and a number of studies have demonstrated the efficacy of this approach [2, 3, 7-12]. The purpose of this paper was to present a case of corneal neovascularization treated with aflibercept. Material and Methods A 30-year-old male with grade 4 right-eye ocular surface burn (Dua’s classi?cation) with involvement of the right eyelid and right facial skin (1% of the body surface) following thermal injury was under observation at the Filatov institute for 18 months. In the early period after burn injury, a free retroauricular graft was performed for right lower eyelid reconstruction; in addition, a conjunctival flap (advancement of the conjunctiva over the cornea) was performed. Subsequently, several medication courses were delivered, and labial mucosal graft was performed for removal of right lower eyelid symblepharon. At the examination immediately prior to aflibercept injection, the patient was diagnosed with lagophthalmos, cicatricial defect of the lower eyelid (the state “after performing the free retroauricular graft”), vascularized corneal opacity (the state “after conjunctival advancement”), and partial lower eyelid symblepharon OD; the left eye was normal. He underwent visual acuity assessment, biomicroscopy, and anterior eye photography and fluorescein angiography (FA) with the fundus camera TRC-50IX (Topcon, Tokyo, Japan). In addition, PhotoM.exe 1.31 software was used to assess the total area of CNV in pixels corresponding to areas of hypofluprescence in FA images (Fig. 2). CNV area measurements were performed before and 6 months after injection. The right eye was treated for CNV with a 0.1 ml (4-mg) subconjunctival injection of aflibercept. Subconjunctival angiogenesis inhibitor injection technique First, the operative field (i.e., the eyelid skin and periocular skin) is cleansed with 0.5% chlorhexidine in alcohol. Second, one drop of ophthalmic 0.5% proparacaine hydrochloride (ALCAINE®, SA Alcon-Couvreur NV, Puurs, Belgium) is instilled into the conjunctival sac three times at 30 second intervals. Third, sterile disposable eye drape is applied. Fourth, the lid speculum is inserted. Fifth, 0.1 ml of aflibercept is injected subconjunctivally at 8 o’clock, 6 mm from the limbus. Sixth, 2% sulfanilamide ointment is applied into the conjunctival sac. Finally, the affected eye is covered with a sterile eye dressing. Results Examination before injection On examination, uncorrected visual acuity (UCVA) was 0.35 OD. Objective examination findings in the right eye included 2-3-mm lagophthalmos; cicatricial defect of the lower eyelid with a pale pink free retroauricular graft; diffuse corneal haze; mild to moderately vascularized cornea; the peripheral cornea partially covered by the advanced conjunctiva; cicatricial changes in the conjunctiva; the inferior conjunctival fornix contracted and partially covered by the labial mucosa; moderately shallow and clear anterior chamber; round pupil with preserved reaction to light; clear crystalline lens (Fig. 1A); and unremarkable fundus.
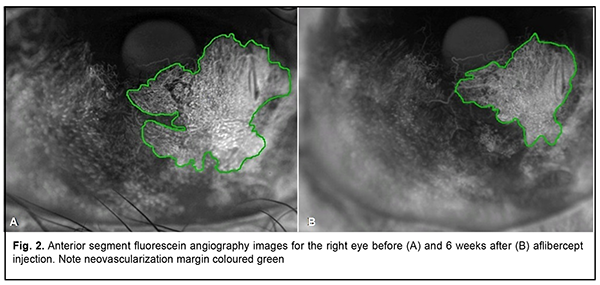
FA showed an area of hyperfluorescence formation corresponding to a corneal neovascularization area of 38,081 pixels (the image was obtained at 16 seconds) (Fig. 2A).
Examination 6 weeks after injection On examination, uncorrected visual acuity (UCVA) was 0.35 OD. Objective examination findings in the right eye included cicatricial defect of the lower eyelid with a pale pink free retroauricular graft; cicatricial changes in the conjunctiva with a pale pink labial mucosa both in the posterior surface of the lower eyelid and the globe; diffuse corneal haze; partially vascularized cornea; reduced CNV area; the peripheral cornea partially covered by the advanced conjunctiva; moderately shallow and clear anterior chamber; round pupil with preserved reaction to light; clear crystalline lens (Fig. 1B); and unremarkable fundus. A FA performed 6 weeks after injection showed reduced hyperfluorescence (a CNV area of 20,782 pixels (the image was obtained at 18 seconds) (Fig. 2B)) compared to previous FA, with a reduction in the CNV area of 17,299 pixels. Discussion Various methods for and approaches to introduction of anti-VEGF agents (an eye-drop approach, subconjunctival, intrastromal, or intraocular injections) in CNV are currently available [2, 3, 7-12]. A retrospective series by Doctor et al [7] aimed to determine whether subconjunctival bevacizumab decreases CNS in patients with ocular surface inflammatory diseases. Patients received 1-3 injections of 2.5 mg subconjunctival bevacizumab, and, thereafter, all 8 eyes of the 7 patients showed a reduction in the neovascularized area. A study by Zaki and Farid [8] included 10 eyes that had CNV caused by factors such as healed corneal ulcers, long-standing chronic inflammatory diseases and corneal ischaemia (caused by contact lenses). All eyes received a single subconjunctival injection of 2.5mg (0.1ml) bevacizumab. As a result, the extent of CNV was significantly decreased as early as 2 weeks post-injection, and the level of CNV continued to decrease noticeably for 3 months. In addition, during the 6-month follow-up, none of the 10 eyes showed any complication that could be related to subconjunctival bevacizumab injection. In the case we presented here, a single subconjunctival injection of 4 mg (0.1ml) aflibercept for thermal injury-induced CNS also had a positive outcome, resulting in a substantial reduction in the CNS area at 6 months. Conclusion In the case reported here, a single subconjunctival injection of 4 mg (0.1ml) aflibercept was effective for reducing area of corneal neovasculatization at 6 months.
References 1.Puchkovskaia NA, Iakimenko SA, Nepomiashchaia VM. [Eye burns]. Moscow: Meditsina; 2001. Russian. 2.Chang JH, Garg NK, Lunde E, et al. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv Ophthalmol. 2012;57(5):415–29. 3.Hashemian MN, Zare MA, Rahimi F, et al. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011 Feb;30(2):215-8. doi: 10.1097/ICO.0b013e3181e291a6. 4.Ulyanov VA, Makarova MB, Molchaniuk NI, et al. [Effect of colloidal silver nanoparticle solution instillation on the ultrastructure of the anterior corneal epithelium and stroma]. J Ophthalmol (Ukraine). 2017; 3:63-9. Russian. 5.Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. 6.Garkava NA, Fedirko PA, Babenko TF, et al. Radiation induced violations of blood circulation in the ciliary body and changes of the anterior chamber angle in the pathogenesis of glaucoma in clean-up workers of the chornobyl NPP accident and residents of contaminated areas. Probl Radiac Med Radiobiol. 2017 Dec;22:332-338. 7.Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008 Oct;27(9):992-5. doi: 10.1097/ICO.0b013e31817786ad. 8.Zaki AA, Farid SF. Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol. Acta Ophthalmol. 2010;88(8):868–71. doi: 10.1111/j.1755-3768.2009.01585. 9.Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res. 2010 Feb;35(2):108-15. doi: 10.3109/02713680903429007. 10.Bock F, Konig Y, Kruse F, et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008 Feb;246(2):281-4. 11.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009 Apr;127(4):381-9. doi: 10.1001/archophthalmol.2009.18. 12.Sarah B, Ibtissam H, Baali Mohammed et al. Intrastromal Injection of Bevacizumab in the Management of Corneal Neovascularization: About 25 Eyes. J Ophthalmol. 2016;2016:6084270. doi: 10.1155/2016/6084270.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|