J.ophthalmol.(Ukraine).2019;6:49-55.
|
http://doi.org/10.31288/oftalmolzh201964955 Received: 17 October 2019; Published on-line: 06 January 2020 Asssociation of vitamin D with autoimmune ophthalmopathy in Graves’ disease Yu.V. Buldygina, Cand Sc (Med); G.M. Terekhova, Cand Sc (Med); L.S. Strafun, Junior Research Fellow; T.V. Fed’ko, Endocrinologist; V.M. Klochkova, Research Fellow; I.I. Savosko, Endocrinologist; Z.G. Lysova, Endocrinologist Komisarenko Institute for Endocrinology and Metabolism of the NAMS of Ukraine; Kyiv (Ukraine) E-mail: Yuliya.buldygina@icloud.com TO CITE THIS ARTICLE: Buldygina YuV, Terekhova GM, Strafun LS, Fed’ko TV, Klochkova VM, Savosko II, Lysova ZG. Asssociation of vitamin D with autoimmune ophthalmopathy in Graves’ disease. J.ophthalmol.(Ukraine).2019;6:49-55. http://doi.org/10.31288/oftalmolzh201964955
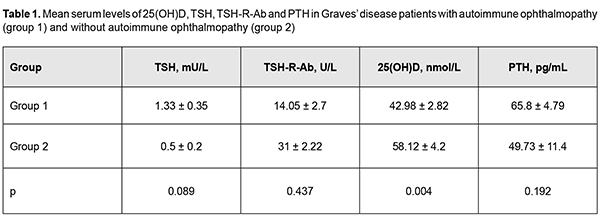
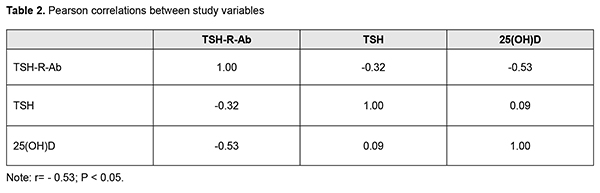
Background: The role of vitamin D in the development of autoimmune thyroid diseases has been actively discussed in the recent literature. Numerous studies have demonstrated an association of low vitamin D levels with autoimmune thyroiditis, with correlations with antithyroid antibody levels, thyroid hormone levels, disease severity and thyroid gland volume, both in adults and in children. However, there are a few relevant reports on Graves’ disease (GD), and only one relevant report is available on GD associated with autoimmune ophthalmopathy (AO). Given that GD complicated by AO is a classic autoimmune disease, it will be interesting to investigate the role of vitamin D in its pathogenesis. Purpose: To examine vitamin D levels and to determine correlations between this characteristic and thyroid-stimulating hormone receptor antibodies (TSH-R-Ab) in patients with Graves’ disease complicated by immune ophthalmopathy. Material and Methods: Serum levels of 25(OH)D, TSH, TSH-R-Ab and parathyroidal hormone (PTH) were measured in 131 patients with GD. Study patients were divided into two groups based on the presence or absence of ophthalmopathy. Group 1 comprised 81 GD patients with AO, and group 2 comprised 50 GD patients without AO. Clinical severity of AO was classified using the NOSPECS. Thirty-two, 30 and 19 patients had class 2b, class 3a or 3b, and class 4a, respectively. Variation statistics with Student t test was used for statistical analyses. The level of significance p ? 0.05 was assumed. Data are presented as mean ± standard error of mean. Pearson correlation analysis was used to identify possible correlations between variables. Results: All patients were euthyroid, and the mean TSH level in group 1 was 1.33±0.35 mU/L, and in group 2, 0.5 ± 0.2 mU/L (Р > 0.05). TSH-R-Ab levels were higher than normal in both groups of patients (group 1: range, 0.8 U/L to 21 U/L , mean value, 14.05± 2.7 U/L ; group 2: range, 1.8 U/L to 14.3 U/L , mean value, 31.0 ± 2.22 U/L; the difference between groups was not significant, р > 0.05). This was anticipated since these abnormalities are pathogenetic in GD. Serum levels of 25(OH)D in both groups were lower than normal (42.98 ± 2.82 nmol/L in group 1 vs 58.12 ± 4.2 nmol/L in group 2; р<0.02). Serum 25(OH)D concentrations were statistically significantly lower in Graves’ disease patients with AO, than in those without ophthalmopathy, and corresponded to vitamin D deficiency and suboptimal status, respectively. There was a significant difference in serum 25(OH)D level, but not in PTH level between the groups. Thus, mean serum PTH level in group 1 was 65.85±4.79 pg/mL, vs 49.73±11.4 pg/mL in group 2 (р > 0.05). TSH-R-Ab levels were negatively linearly correlated with the serum 25(OH)D level in the study cohort (elevated TSH-R-Ab levels correlated with low 25(OH)D levels). Conclusion: First, there was no statistically significant difference in TSH-R-Ab levels between Graves’ disease patients with AO and those without AO (14.05±2.7 U/L and 31±2.22 U/L, respectively; р > 0.05). Second, the mean serum 25(OH)D level for the study cohort of Graves’ disease patients (47.63 ± 2.48 nmol/L) was insufficient, and the mean level for patients with AO was significantly lower than the mean level for patients without AO (42.98 ± 2.82 nmol/L vs 58.12 ± 4.2 nmol/L; р<0.02). Finally, there was a negative correlation of TSH-R-Ab levels with 25(OH)D levels in Graves disease patients with AO (r= - 0.53; р<0.05). Keywords: autoimmune ophthalmopathy, Graves’ disease, vitamin D
Introduction Autoimmune ophthalmopathy (AO; Graves’ ophthalmopathy, endocrine orbitopathy, malignant exophthalmos, thyrotoxic ophthalmopathy) is an autoimmune disease characterized by a complex orbital tissue injury and accompanied by infiltration, edema and proliferation of the retrobulbar adipose tissue, muscles and connective tissue. The pathogenesis of AO is closely linked with autoimmune thyroid diseases (AITD); AO may be associated with Graves’ disease (GD) in the majority (about 90%) of cases or with Hashimoto’s thyroiditis in 10% of cases, or may run a course not associated with thyroid disease. AO is seen in 5% to 20% of patients with GD [1], has a female to male ratio of about 4:1 and a peak incidence between 40 and 60 years [2]. GD is a systemic autoimmune disorder characterized by the infiltration of thyroid antigen-specific T cells into thyroid-stimulating hormone receptor (TSH-R)-e-pressing tissues. Stimulatory autoantibodies (Ab) in GD activate the TSH-R leading to thyroid hyperplasia and hyperfunction [3]. In Graves’ ophthalmopathy (GO), orbital fibroblasts are the autoimmune target cells and express relatively high levels of functional TSH-R. Stimulating Ab bind to TSH-R that also interacts with IGF1 receptors (IGF1-R) on the surface of thyrocytes and on orbital fibroblasts, which results in the TSH-R-Ab interaction with TSH-R activating both IGF1R downstream pathways and TSH-R signaling [3, 4]. There have been reports [5, 6] of numerous potential factors (genetic susceptibility; external triggers like iodine, selenium, and interferon- and iodine-containing drugs; exposure to radiation; cigarette smoking; viral infections; stress and chemicals) influencing initiation and progression of AITD. In recent decades, there have been studies on non-classic effects of vitamin D, particularly, on its role in regulation of cell proliferation and differentiation and immune modulation, since vitamin D deficiency has been reported in several chronic conditions associated with increased inflammation and deregulation of the immune system [7, 8, 9, 10]. It is well-established that vitamin D can affect antigen-presenting cells (APC), while its deficiency can impair the function of cells of acquired and innate immunity [11]. It is likely that it suppresses the development of autoimmune reactions and produces an anti-inflammatory effect by provoking differentiation of dendritic and regulating T cells and decreasing Th17 response and inflammatory cytokine secretion [6, 12]. Although it has been reported that vitamin D deficiency may be associated with increased risk for progression of AITD, it is still not understood whether this has a role in disease pathogenesis or is a consequence of the disease. Biological action of vitamin D is mediated by its active form, 1,25(OH)2D3. In target tissues, 1,25(OH)2D, an active form of vitamin D, exerts its action by associating with a specific nuclear vitamin D receptor (VDR), a member of a rather large family of nuclear hormone receptors [13]. Vitamin D in the form of 1,25(OH)2D is involved, either directly or indirectly, in activation or suppression of as many as 200-500 genes, representing about 3-5 % of the human genome, and including those responsible for regulation of cell proliferation, differentiation, and apoptosis and angiogenesis [14]. 1,25(OH)2D3 receptors have been identified in more than 35 organs and tissues. Cells of the intestine, bone tissue, kidneys, brain tissue, prostate and breast, as well as immune cells (including B and T cells, monocytes, macrophages and dendritic cells) express VDR and respond to 1,25(OH)2D [6,13]. In addition, immune cells exhibit an active vitamin-D metabolism, and some of them can produce 1?-hydroxylase, the enzyme required to convert 25(OH)D3 to 1,25(OH)2D3. The ability of immune cells to metabolize vitamin D ensures a physiological high concentration of active 1,25(OH)2D3 in a local lymphoid environment, which promotes its speci?c action and limits any undesirable high concentration-related systemic effects [6, 8,11,12]. The literature has identified many potential factors at play for the initiation and progression of AITD. Some of these factors, including tumor necrosis factor-alpha (TNF-?), and interleukin (IL)-6, contribute to thyroid inflammation, and vitamin D is an additional factor [15]. Vitamin D receptor has been reported to exist in ocular barrier cells and vitamin D has been reported to enhance corneal epithelial barrier function through regulating gap junctions and tight junction. In mice, vitamin D has been shown to suppress ocular surface inflammation by inhibiting Langerhans cell migration into corneas, thus inhibiting corneal neovascularization [16]. Studies have reported contradictory results regarding an association between vitamin D deficiency and dry eye syndrome (DES), a common ocular condition found in 14-15% of population aged 50 and over and significantly affecting the quality of life. It is known that 65%-85% of patients with AO have symptoms of DES. A cross-sectional analysis study by Yoon et al [16] involved 17,542 Korean adults, found that the deficient sunlight exposure and low serum 25(OH)D levels were a risk factor for DES, but symptomatic DES was associated only with deficient serum 25(OH)D levels (<12 ng/mL) in Korean adults. Some later studies [17, 18] found similar results. Several studies [19, 20], however, reported no significant associations between serum vitamin D levels and DES. Although the complete explanation for the mechanism through which Graves’ opthalmopathy leads to DES is not entirely known, it is most likely that multiple mechanisms are involved and have a synergistic effect. Known mechanisms include tear film dysfunction due to increased evaporation and/or ocular inflammation, aberrant stimulation of lacrimal glands leading to hyposecretion, and other currently unknown mechanisms currently combining to cause the symptoms and signs of DES [21]. Several gene studies demonstrated associations of polymorphisms in the VDR gene, vitamin D binding protein (VDP) gene, 1-alpha-hydroxylase gene, and 25-hydroxylase gene with susceptibility to autoimmune abnormalities. However, some other studies did not confirm these associations. Recent analyses of the literature on the role of vitamin D in thyroid diseases [6, 10, 13, 14, 22] have demonstrated associations of vitamin D deficiency with Hashimoto’s thyroiditis, with or without correlations with antithyroid antibody levels, thyroid hormone levels, disease severity and thyroid gland volume [23, 24, 25, 26, 27], both in adults and in children [23]. Although potential associations between vitamin D and GD were investigated less than those between vitamin D and Hashimoto’s thyroiditis, the majority of studies reported on low vitamin D levels in patients with GD, and on correlations of vitamin D levels with antithyroid antibody and thyroid hormone levels [31, 32, 33, 34, 35, 36]. Some other studies, however, either found no association between low vitamin D levels and AITD in adults [6, 9, 23] and in children [37], or found associations only for specific subgroups [6, 38]. In a study by Planck et al [33], the prevalence of vitamin D deficiency and insufficiency was higher in 292 patients with newly diagnosed GD compared to controls. The study, however, did not observe any correlation between the vitamin D levels and levels of fT4, fT3, TRAb, and TPOAb and relapse within 1 year after terminating treatment with antithyroid drugs. In addition, Graves’ disease patients without Graves’ opthalmopathy at diagnosis had the same vitamin D levels as those with Graves’ opthalmopathy. Moreover, there was no difference in the vitamin D levels at baseline between individuals who achieved remission and those who relapsed. Those authors [33] stressed that theirs was the first study investigating the relationship between vitamin D levels and GO, showing no association between vitamin D levels with the presence of GO at the onset of GD. Contradictions in the literature and the absence of well-grounded conclusions can be partially explained by limitations in study design, seasonal variations in blood vitamin D levels, and intra-assay analytical variability affecting the results for measurements of vitamin D levels. In addition, differences in definitions of vitamin D deficiency/insufficiency have contributed to contradictory results. Therefore, patients with Hashimoto’s thyroiditis and Graves’ disease in AO have significantly impaired cell-based and humoral immunity during the course of the disease. Although some aspects of the pathogenesis are yet to be fully elucidated, recent studies have demonstrated that immune correction may be promising in patients with these disorders. Given the contradictory data on the effect of 25(OH)D on the acquired immune system, further randomized controlled trials are needed to clarify (a) the role of vitamin D as an immunomodulator and (b) its potential benefits in the treatment of AITD and AO. The purpose of this study was to examine vitamin D levels and to determine correlations between this characteristic and thyroid-stimulating hormone receptor antibodies (TSH-R-Ab) in patients with Graves’ disease complicated by immune ophthalmopathy. Materials and Methods 25-hydroxyvitamin D (25(OH)D) is widely regarded as the best indicator of vitamin D status and a marker of vitamin D deficiency and concomitant calcium metabolism disorder. Serum 25(OH)D concentrations were measured in nmol/L using enzyme-linked immunosorbent assay (ELISA; ADVIA Centaur XP System, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY). Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe recommend pharmacological therapy for vitamin D deficiency [serum 25(OH)D < 50 nmol/L), and define suboptimal status and target concentration for optimal vitamin D effects as 50-75 nmol/L and 75-125 nmol/L, respectively [38]. Parathyroid hormone (PTH) concentrations were assessed as a marker of impaired functional parathyroid activity with the development of secondary hyperparathyroidism as a classical physiological response to vitamin D deficiency in the body. Serum PTH concentrations were measured using chemiluminescent immunoassay kits (reference PTH range, 15 pg/mL to 65 pg/mL). Serum TSH concentrations were measured using radioimmunoassay kits (Immunotech, Prague, Czech Republic; reference TSH range, 0.17 mU/L to 4.05 mU/L) to examine thyroid function. Serum concentrations of antibodies to TSH-R (TSH-R-Ab), a major marker of the autoimmune process in Graves’ disease, were measured using ELISA kits (reference TSH-R-Ab ? 1.5 U/L). Variation statistics with Student t test was used for statistical analyses. The level of significance p ? 0.05 was assumed. Data are presented as mean ± standard error of mean. Pearson correlation analysis was used to identify possible correlations between parameters. We prospectively analyzed 131 Graves’ disease patients (119 women and 12 men; age, 29 years to 62 years; mean age, 46±8 years) who were treated or observed at the Department of General Endocrine Pathology, Komisarenko Institute for Endocrinology and Metabolism. At baseline, mean serum TSH, 25(OH)D, TSH-R-Ab and PTH levels for the study cohort were 1.00 ± 0.23 mU/L, 47.63 ± 2.48 nMol/L, 12.97 ± 1.95 U/L , and 63.17 ± 4.52 pg/mL, respectively. Study patients were divided into two groups based on the presence or absence of ophthalmopathy. Group 1 comprised 81 Graves’ disease patients with AO, and group 2 comprised 50 Graves’ disease patients without AO. Clinical severity of AO was classified using the NOSPECS. Thirty two, 30 and 19 patients had class 2b, class 3a or 3b, and class 4a, respectively. Of the 50 patients of the group of patients without AO, five had NOSPECS class 1a or class 1b. These five patients were not included in the group of patients with AO, since such changes are considered not manifestations of autoimmune ophthalopathy, but resulting from stimulation of the sympathetic nervous system by excessive hormones. Results and Discussion All patients were euthyroid receiving (a) thyroid hormone replacement therapy after thyroidectomy or (b) antithyroid therapy (merkazolil and/or thyrosol), and the mean TSH level in group 1 was 1.33±0.35 mU/L, and in group 2, 0.5±0.2 mU/L (Р > 0.05). It should be noted that TSH-R-Ab levels were higher than normal in both groups of patients (group 1: range, 0.8 U/L to 21 U/L, mean value, 14.0 5± 2.7 U/L; group 2: range, 1.8 U/L to 14.3 U/L, mean value, 31.0 ± 2.22 U/L; the difference between groups was not significant, р > 0.05). This was anticipated, since these abnormalities are pathogenetic in the disease, and the pathogenesis is based on the production of antibodies that bind to and activate TSH-R on the membrane of thyrocytes, and the activation process further develops, resulting in increased iodine capture, increased production of thyroid peroxidase and thyroglobulin, and, eventually, in thyroid hyperfunction. Serum levels of 25(OH)D in both groups were lower than normal (42.98 ± 2.82 nmol/L in group 1 vs 58.12 ± 4.2 nmol/L in group 2; р<0.02). Serum 25(OH)D levels were statistically significantly lower in Graves’ disease patients with AO, than in those without ophthalmopathy, and corresponded to vitamin D deficiency and suboptimal status, respectively. Interestingly, there was a significant difference in serum 25(OH)D level, but not in PTH level between the groups. Thus, mean serum PTH level in group 1 was 65.85±4.79 pg/mL, vs 49.73±11.4 pg/mL in group 2 (р > 0.05). It should be noted that the mean serum PTH level in group 1 was at the upper limit of the normal range, pointing to the tendency towards a compensatory increase in parathyroid function in response to deficiency in 25(OH)D among Graves’ disease patients with AO. The above characteristics are presented in Table 1.
Thereafter, Pearson correlation analysis was used to identify possible correlations between parameters (Table 2).
Our study showed that TSH-R-Ab levels were negatively linearly correlated with the serum 25(OH)D level in the study cohort (elevated TSH-R-Ab levels correlated with low 25(OH)D levels). Our findings are in agreement with the results of other studies regarding correlation of elevated levels of antithyroid antibodies with vitamin D deficiency in autoimmune thyroid diseases. As opposed to the study by Planck et al [33], we found not only deficient serum 25(OH)D levels in Graves’ disease patients with AO compared to those without AO, but also a negative correlation of TSH-R-Ab levels with 25(OH)D levels, which points to the role of the latter in autoimmune processes in AO. Conclusion First, there was no statistically significant difference in TSH-R-Ab levels between Graves’ disease patients with AO and those without AO (14.05 ± 2.7 U/L and 31 ± 2.22 U/L, respectively; р > 0.05). Second, the mean serum 25(OH)D level for the study cohort of Graves’ disease patients (47.63 ± 2.48 nmol/L) was insufficient, and the mean level for patients with AO was significantly lower than the mean level for patients without AO (42.98 ± 2.82 nmol/L vs 58.12 ± 4.2 nmol/L; р<0.02). Finally, there was a negative correlation of TSH-R-Ab levels with 25(OH)D levels in Graves disease patients with AO (r= - 0.53; р<0.05). References 1.Oliinyk VA, Terekhovа HM, Buldygina YuV, Fed’ko TV, Klochkova VM, Rakov OV, Lysova ZH. [Treatment of autoimmune ophthalmopathy in patients with diffuse toxic goiter by glucocorticoids]. Endokrynologiia. 2017;22(2):108–14. Ukrainian. 2.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9–26. 3.Kahaly GJ, Bartalena L, Heged?s L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves' Hyperthyroidism. Eur Thyroid J. 2018 Aug;7(4):167–86. 4.Smith TJ, Janssen JAMJL. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019 Feb 1;40(1):236–67. 5.Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. 2017 Sep; 18(9): 1949. 6.Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental issues in thyroid diseases. Front Endocrinol (Lausanne). 2017;8:50. 7.Nettore IC, Albano L, Ungaro P, Colao A, Macchia PE. Sunshine vitamin and thyroid. Rev Endocr Metab Disord. 2017 Jan14; 18(3). 8.Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018 Nov 3; 10(11): pii: E1656. 9.D'Aurizio F, Villalta D, Metus P, Doretto P, Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2015 May;14(5):363–9. 10.Muscogiuri G, Tirabassi G, Bizzaro G, Orio F, Paschou SA, Vryonidou A, et al. Vitamin D and thyroid disease: to D or not to D? Eur J Clin Nutr. 2015 Mar;69(3):291–96. 11.Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2018; 9: 3160. 12.Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. 2018 Jun; 19(6): pii: E1618. 13.Chekman IS, Gorchakova NO, Berezhniy VV, Davydiuk AV, Roman’ko MR. [Pharmacology of vitamin D]. Sovremennaia pediatriia. 2017; 2(82):28–36. Ukrainian. 14.Vondra K, St?rka L, Hampl R. Vitamin D and thyroid diseases. Physiol Res. 2015;64 Suppl 2:95–100. 15.Merrill SJ, Minucci SB. Thyroid autoimmunity: an interplay of factors. Vitam Horm. 2018;106:129–45. 16.Yoon SY, Bae SH, Shin YJ, Park ShG, Hwang S-H, Hyon JY, Wee WR. Low Serum 25-Hydroxyvitamin D Levels Are Associated with Dry Eye Syndrome. PLoS One. 2016; 11(1): e0147847. 17.Meng YF, Lu J, Xing Q, Tao JJ, Xiao P. Lower serum vitamin D level was associated with risk of dry eye syndrome. Med Sci Monit. 2017 May 10;23:2211–6. 18.Jin KW, Ro JW, ShinYJ, Hyon JY. Correlation of vitamin D levels with tear film stability and secretion in patients with dry eye syndrome. Acta Ophthalmol. 2017 May; 95(3):e230-e235. 19.Jeon D-H, Yeom H, Yang J, Song JS, Lee HK, Kim HCh. Are serum vitamin D levels associated with dry eye disease? Results from the Study Group for Environmental Eye Disease. J Prev Med Public Health. 2017 Nov;50(6):369–76. 20.Jee D, Kang S, Yuan Ch, Eunyoung Cho E, Arroyo JG, and The Epidemiologic survey Committee of the Korean Ophthalmologic Society. Serum 25-hydroxyvitamin D levels and dry eye syndrome: differential effects of vitamin D on ocular diseases. PLoS One. 2016; 11(2): e0149294. 21.Selter JH, Gire AI, Sikder S. The relationship between Graves’ ophthalmopathy and dry eye syndrome. Clin Ophthalmol. 2015; 9: 57–62. 22.Wang J, Lv S, Chen G, Gao C, He J, Zhong H, Xu Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015 Apr 3;7(4):2485–98. 23.Altieri B, Muscogiuri G, Barrea L, Mathieu C, Vallone CV, Mascitelli L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord. 2017 Sep;18(3):335–46. 24.Chaudhary S, Dutta D, Kumar M, Saha S, Mondal SA, Kumar A, Mukhopadhyay S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J Endocrinol Metab. 2016 May-Jun; 20(3): 391–8. 25.Simsek Y, Cakir I, Yetmis M, Dizdar OS, Baspinar O, Gokay F. Effects of vitamin D treatment on thyroid autoimmunity. J Res Med Sci. 2016; 21(6): 85. 26.Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, Khalili D, Vakili AZ, Azizi F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto's thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019 May;51(5):296–301. 27.Krysiak R, Kowalcze K, Okopie? B. Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto's thyroiditis and low vitamin D status. Pharmacol Rep. 2019 Apr;71(2):367–73. 28.Kivity Sh, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dank? K, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011 May; 8(3): 243–7. 29.Zhang H, Liang L, Xie Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves disease. Endocr Pract. 2015 Mar;21(3):258–63. 30.Muscogiuri G, Mari D, Prolo S, Fatti LM, Cantone MC, Garagnani P, et al. 25 hydroxyvitamin D deficiency and its relationship to autoimmune thyroid disease in the elderly. Int J Environ Res Public Health. 2016 Aug 26;13(9). 31.Yasuda T, Okamoto Y, Hamada N, Miyashit K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine. 2012 Dec;42(3):739–41. 32.Xu M-Y, Cao B, Yin J, Wang D-F, Chen K-L, Lu Q-B. Vitamin D and Graves’ disease: a meta-analysis update. Nutrients. 2015 May; 7(5): 3813–27. 33.Planck T, Shahida B, Malm J, Manjer J. Vitamin D in Graves Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur Thyroid J. 2018 Jan;7(1):27-33. 34.Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine. 2013 Feb; 43(1): 230–2. 35.Ma J, Wu D, Li C, Fan C, Chao N, Liu J, et al. Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine (Baltimore). 2015 Sep;94(39):e1639. 36.Mangaraj S, Choudhury AK, Swain BM, Sarangi PK, Mohanty BK, Baliarsinha AK. Evaluation of vitamin D status and its impact on thyroid related parameters in new onset Graves' disease- A cross-sectional observational study. Indian J Endocr Metab. 2019;23:35–9. 37.Ke W, Sun T, Zhang Y, He L, Wu Q, Liu J, Zha B. 25-hydroxyvitamin D serum level in Hashimoto's thyroiditis, but not Graves' disease is relatively deficient. Endocr J. 2017 Jun 29;64(6):581–7. 38.P?udowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sok?? D, Czech-Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319–27.
Conflict of Interest: The authors declare that there are no conflicts of interest regarding this study and the publication of this article.
|