J.ophthalmol.(Ukraine).2019;6:23-28.
|
http://doi.org/10.31288/oftalmolzh201962328 Received: 31 October 2019; Published on-line: 06 January 2020 Meibomian gland dysfunction accompanied by palpebral demodicosis in patients with type 2 diabetes mellitus T.M. Zhmud,1 Cand Sc (Med); G.I. Drozhzhyna,2 Dr Sc (Med), Prof. 1 Pirogov Vinnytsia National Medical University; Vinnytsia (Ukraine) 2 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: Gtatyana@email.ua TO CITE THIS ARTICLE: Zhmud TM, Drozhzhina GI. Meibomian gland dysfunction accompanied by palpebral demodicosis in patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2019;6:23-28. http://doi.org/10.31288/oftalmolzh201962328 Background: Demodicosis is one of the most common dermatoses. Demodex blepharitis accounts for 39% to 88% of all cases of inflammatory eyelid disorders. Purpose: To assess the prevalence of meibomian gland dysfunction (MGD) accompanied by palpebral demodicosis in patients with type 2 diabetes mellitus (T2DM). Material and Methods: Seventy-five patients (150 eyes; mean age, 54 ± 8 years; 34 men (45.3%) and 41 women (54.7%)) with compensated T2DM and symptoms of MGD were included in this study. Patients underwent visual acuity assessment, biomicroscopy, ophthalmoscopy, compression test (to assess meibomian gland secretions), meibography, Schirmer test, tear film breakup time, and laboratory test for Demodex mites, and laboratory studies (lipidogram, fasting blood sugar, and glycated hemoglobin). In addition, they were administered the Ocular Surface Disease Index (OSDI) questionnaire. Moreover, diabetes duration, blood sugar levels, and type of therapy for diabetes were assessed. Results: Palpebral demodicosis was twice as common in patients with T2DM duration more than 10 years as in those with T2DM duration less than 10 years (p = 0.002). Our meibography study found changes in meibomian glands in 90% of diabetics with palpebral demodicosis, with the mean meibograde score of 5.0 ± 0.9 points, which indicated a predominance of moderate MGD. Conclusion: Metabolic defects in patients with T2DM contribute to the development of palpebral demodicosis that was found in 61.3% of patients. Mixed type dry eye disease was found in T2DM patients with palpebral demodicosis, which required treatment for MGD and restoration of the tear film aqueous layer. Keywords: palpebral demodicosis, meibomian gland dysfunction, type 2 diabetes mellitus
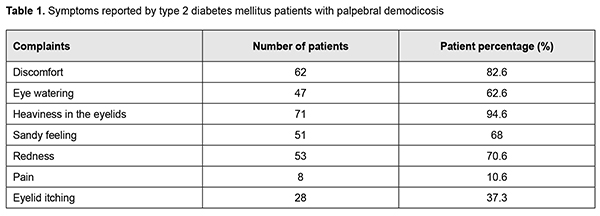
Introduction Demodicosis is one of the most common dermatoses. Demodex blepharitis accounts for 39% to 88% of all cases of inflammatory eyelid disorders. Eighty-nine per cent of patients with the disease were found to be parasite carriers, although most parasite carriers exhibit no manifestations of the disease [1]. The following causes of the development of meibomian gland dysfunction (MGD) have been identified: age-related changes; gastrointestinal disorders; seborrheic dermatitis, acne rosacea, diabetes mellitus, Sj?gren syndrome, rheumatoid arthritis, psoriasis, atopic dermatitis, and hormone-related changes [2, 3]. Patients with MGD have a decreased number of normally functioning meibomian glands (MG) due to MG orifice metaplasia [4]. Administration of systemic medications (e.g., exposure to retinoic acid derivatives that are frequently used in the management of acne), hormone replacement therapy in menopausal women, antihistamines and antidepressants may also contribute to the development of the disease [5, 6]. Demodex infestation of the eyelids has been found to develop in the presence of impaired local immunity and secondary immune deficiency [7]. The prevalence of MGD increases with age [1, 8, 9]. Demodex mites have been found in all races and ethnic groups of the human population. No association of Demodex infestation with gender was found in a study by Beirnat and colleagues [10]. No significant differences regarding Demodex spp. prevalence were noted between women and men (42.1% and 39.7% respectively) (?2 = 0.09, p = 0.7) [11]. The prevalence of Demodex brevis increases with age, whereas that of Demodex folliculorum does not change with age [12]. Meibomian gland dysfunction is caused primarily by terminal duct obstruction with thickened opaque meibum containing keratinized cell material [9, 13]. Although the hair follicle mites of the genus Demodex (Demodecidae) were first discovered in humans as early as 1841, their pathogenic role in human disease is controversial. Some investigators consider them symbionts [1, 9], while others believe that they have a potential pathogenic role under severe conditions for the host [14]. Mites of the genus Demodex (Demodecidae) are obligate parasites that feed and breed in follicles of the meibomian and other sebaceous glands, and exert multidimensional effects on infested human tissues like mechanical and chemical tissue irritation, impaired symbiosis of pathogenic microflora, MGD, dysfunction of the glands of Zeis, impaired tear film lipid layer, para- or hyperkeratosis, and local immune suppression and sensibilization [1, 15]. The pathogenesis of posterior blepharitis is based on the primary, chronic inflammation of the meibomian glands due to qualitative impairment of meibomian gland secretion [16]. Chronic diseases like malignant tumors, diabetes mellitus, and renal insufficiency are known to directly affect immunity. Skin invasions by Demodex progress in disorders affecting humoral and cell-mediated immunity. Whether Demodex infestation develops or not depends on the internal factors like sebaceous gland dysfunction and T-cell suppression, as well as on specific external factors. G?k?e and colleagues [17, 18] found infestation by D. folliculorum in 24.6% of 69 patients with type 2 diabetes mellitus (T2DM) and concluded that these findings suggest that poor blood glucose regulation increases the susceptibility to D. folliculorum mite infestation in patients with T2DM. In the study by Keskin Kurt and co-authors [19], pregnant women with gestational diabetes had a statistically significantly higher Demodex density compared to pregnant women without gestational diabetes (24.2 vs. 3.3%; p < 0.001). Manifestations of demodicosis are believed to be associated with immune deficiency conditions. A high density of Demodex mites has been found in inflammatory skin sites of patients with HIV infection or cancer and immunosuppressive hemodialysis subjects with both chronic renal insufficiency and diabetes mellitus. Kim et al [15] demonstrated that after treatment, Demodex was nearly eradicated, tear concentrations of IL-1? and IL-17 were significantly reduced and substantial clinical improvement was observed in all patients. They believe that the pathogenesis of demodicosis (particularly, palpebral demodicosis) may be associated with hypersensitive immune responses on the ocular surface and lid tissues [15, 17]. Bacterial antigens on the mite surface can trigger a host inflammatory response, while bacteria present in the parasite intestine may stimulate proliferation of mononuclear cells in the peripheral blood of infected persons. Demodex mites can also transmit viruses and fungi [11]. In addition, they may cause peripheral corneal neovascularization, corneal infiltration, phlyctenular corneal disease, corneal infiltration, superficial corneal opacity, corneal nodular opacity, etc. [20]. Three provoking factors are required for the population of Demodex to increase: a good tissue blood supply, poor eyelid hygiene, and low immunity status [17]. Identifying etiopathogenetic relationships of altered lipid metabolism and meibomian gland dysfunction with the development of dry eye disease is of primary importance for effective treatment and improved quality of life of patients with diabetes mellitus. Although a variety of disease-specific and symptomatic medications are available, available treatments of palpebral demodicosis are still insufficiently effective. This is contributed to by a long therapy duration, noncompliance with hygiene requirements and treatment regimens, administration of symptomatic non-acaricidal medications, absence of adequate treatment for the chronic disease, and the requirement for comprehensive treatment (palpebral demodicosis should be treated by the ophthalmologist, while face skin demodicosis, by the dermatologist) [21]. Poor knowledge on the etiopathogenesis and inadequate efficacy of therapeutic treatments for demodicosis contribute to chronic disease with relapses and severe clinical manifestations, which is an important factor of psychic trauma in patients. This can result in the development of psychasthenic conditions and neuroses and demonstrates the medical and social value of the disease. Palpebral demodicosis can be diagnosed by several methods. The laboratory test is thought to be the simplest method. The principle is as follows. Eyelashes are epilated, placed on microscope slides with some potassium hydroxide 10% solution, and the total number of Demodex is counted. In vivo laser scanning confocal microscopy has been found to be better than traditional methods in detecting the number and sensitivity of Demodex [22]. As impaired metabolism and low immunity contribute to the onset of symptoms of irritation, organic changes in the eyelid margin, and activation of opportunistic flora in patients with type 2 diabetes mellitus, investigating etiopathogenetic factors of the development of MGD is of primary importance for the effective treatment and improved quality of life in these patients. The purpose of the study was to assess the prevalence of meibomian gland dysfunction associated with palpebral demodicosis in patients with type 2 diabetes mellitus. Material and Methods Seventy-five patients (150 eyes; mean age, 54 ± 8 years; 34 men (45.3%) and 41 women (54.7%); mean diabetes duration, 12 ± 4 years) with compensated type 2 diabetes mellitus and symptoms of MGD were included in this study. Patients underwent visual acuity assessment, biomicroscopy, ophthalmoscopy, compression test (to assess meibomian gland secretions) [1], meibography, Schirmer test, tear film breakup time, laboratory test for Demodex mites, and laboratory studies (lipidogram, fasting blood sugar, and glycated hemoglobin). In addition, they were administered the Ocular Surface Disease Index (OSDI) questionnaire to evaluate dry eye disease-related symptoms. Moreover, diabetes duration, blood sugar levels, and type of therapy for diabetes (insulin or anti-diabetic drugs) were assessed. Meibography is a technique for observing and documenting the morphology of meibomian glands in vivo. Different contact and non-contact meibography techniques are available [23]. In our practice, we use a device that we have developed (“a portative device for investigating meibomian gland status”), which is based on making photographs of everted eyelids in the infrared (IR) spectrum (patent No. 126656, “A method for obtaining meibomian gland images”, 2018). The device employs the Sony Alpha DSLR-A500 camera with a removed blocking IR filter. The camera lens is additionally equipped with nine battery-powered 850-nm IR diodes and 720-nm IR filter for blocking visible light [24]. Each eyelid was assigned a "meibograde" based on the obtained data. Essentially, the meibograde comprises three distinct categories based on previously described histopathologic changes in the meibomian glands: gland distortion, gland shortening, and gland dropout [9]. Each category was scored 0-3 based on the area of eyelid involved: score 0, no area involved; 1, < 33% involved; 2, 33%-66% involved; 3, >66% of area involved [23]. Statistical analyses were conducted using Statistica 8 (StatSoft, Tulsa, OK, USA) software. Pearson Chi-square was used to assess differences between groups. Results Of the 75 study patients with T2DM, 46 (61.3%) were found to have palpebral demodicosis. The major complaints were ocular discomfort, eye watering, heaviness in the eyelids, sandy feeling, redness, ocular pain, and itching. The commonest complaint was heaviness in the eyelids (94.6%), followed by ocular discomfort (82.6%), periodic redness, mostly in the evening (70.6%), sandy feeling (68%) and eye watering (62.6%) (Table 1).
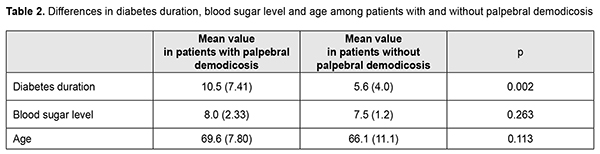
Forty-five subjects (60%) had an OSDI score greater than 15, indicating dry eye symptoms. Palpebral demodicosis was almost as common among men as it was among women (45.7% vs 54.3%, respectively; p = 0.95). There was no statistically significant difference in blood sugar levels or age between patients with and without palpebral demodicosis. Palpebral demodicosis was twice as common in patients with T2DM duration more than 10 years as in those with diabetes duration less than 10 years (Table 2). Diabetes duration was statistically significantly longer in diabetics with palpebral demodicosis than in those without palpebral demodicosis (p = 0.002). Thus, mean diabetes duration was 10.5 years and 5.6 years for patients with palpebral demodicosis and those without palpebral demodicosis, respectively.
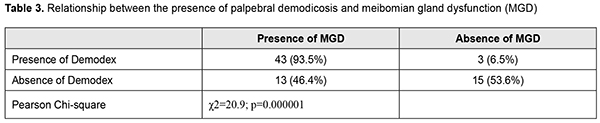
Among diabetics with palpebral demodicosis, 21 (45.7%) were on insulin treatment, and 25 (54.4%) were on anti-diabetic drugs. Among diabetics without palpebral demodicosis, 8 (28.6%) were on insulin treatment, and 20 (71.4%) were on anti-diabetic drugs. No significant relationship was found between the type of therapy for diabetes and presence of palpebral demodicosis (p = 0.14). Table 3 presents data on the presence of meibomian gland dysfunction and the presence of palpebral demodicosis in patients with T2DM.
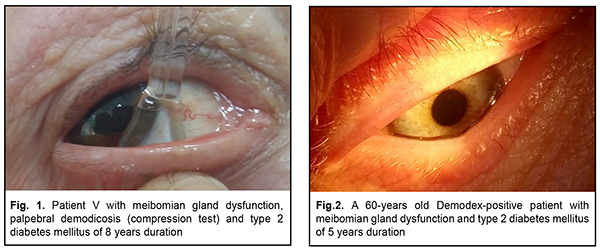
There was a statistically significant association between the presence of palpebral demodicosis and the presence of MGD (?2=20.9; р=0.000001). Thus, MGD was found in 93.5% of diabetics with diagnosed palpebral demodicosis, and only in 46.4% of those without palpebral demodicosis. In diabetics with palpebral demodicosis, mean tear production as assessed by the Schirmer test was as low as 6.9 ± 0.1 mm/5 min, and mean tear film break-up time was as short as 8.4 ± 0.5 s. Meibomian gland dysfunction was observed in 31 patients (41.3%) (Fig. 1), and was characterized by decreased numbers of normally functioning meibomian glands, and altered meibomian secretion (Fig. 2). Functional meibomian gland characteristics correlated with duration of diabetes shorter than 5 years (r1=-0.68, r2=-0.56).
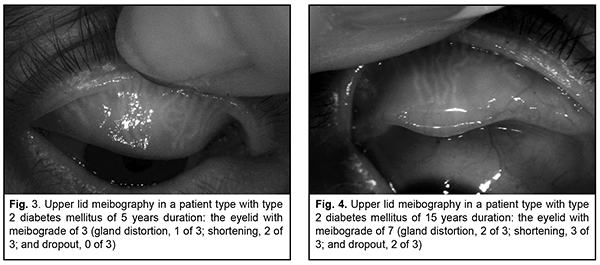
Our meibography study found changes in meibomian glands in 90% of diabetics with palpebral demodicosis. In these patients, the mean meibograde score was 5.0 ± 0.9 points, which indicated a predominance of moderate MGD (Fig. 3). Interestingly, severe MGD was found in 14% of patients with duration of diabetes of more than 5 years (Fig. 4).
Discussion More than half of patients in our study, 48 (64%), were positive for Demodex mites, which is almost 2.5-fold more than in the study by Gokce et al [17], in which D. folliculorum mite infestations were found in 17 (24.6%) of 69 patients. Gokce et al [17] suggest that poor blood glucose regulation increases the susceptibility to Demodex mite infestation in patients with type 2 diabetes. Several abnormalities might contribute to the increased susceptibility and severity of infections in diabetic patients, including lower chemotactic activity of neutrophils, poor leukocyte-endothelial cell interactions and decreased quantity of leukocytes in inflammatory lesions, and a reduction in lymph node retention capacity and reduced release of cytokines, such as tumor necrosis factor-alpha, interleukins and prostaglandins [25]. Each Demodex-positive patient had dry eye syndrome (DES) and signs of MGD as assessed by the compression test and objective symptoms of the disease. Studies by Tomlinson [9] and Qiao and Yan [21] demonstrated a significant association between the degree of DES and MGD. They believe that the main cause of DES is excessive evaporation of the tear film aqueous due to deficient tear film lipid layer [22]. Pathan [26] concluded that (1) the prevalence of MGD in the diabetic population was 56% which is more than the general population prevalence, and (2) MGD is an important predisposer for severe diseases like dry eye in this subgroup of patients. Individuals older than 50 years exhibit changes in meibomian glands and ducts, with gland distortion and narrowing of the duct orifice, which may result in a reduced delivery of meibum to the tear film lipid layer. Low meibum delivery and changes in oil composition can lead to tear film instability, increased tear evaporation and ultimately to evaporative dry eye [27]. In the current study, we also observed changes in meibomian gland ducts, tear film instability and reduced tear production in our T2DM patients with demodex infestation, indicating the mixed type of dry eye disease as per TFOS DEWS II Report Executive Summary [27]. In addition, we used a laboratory method with light microscopy for diagnosing demodex infestation. A recent (2019) study by Wang and colleagues [22], however, demonstrated that in vivo laser scanning confocal microscopy (IVCM) had better sensitivity for palpebral demodicosis compared to light microscopy (100% (95% СІ 97,02-100%) vs 56.69% (95% CI 48.55-64.49%)). Given the findings of this study and the chronic and progressive course of diabetes mellitus, patients with T2DM should be recommended to undergo examinations and receive courses of treatment periodically to improve their quality of life. Conclusion First, in patients with T2DM, metabolic deficits contribute to the development of palpebral demodicosis that we found in 61.3% of diabetic patients. Second, palpebral demodicosis was twice as common in patients with T2DM duration of more than 10 years as in those with diabetes duration less than 10 years (p = 0.002). Third, our meibography study found changes in meibomian glands in 90% of diabetics with palpebral demodicosis, with the mean meibograde score of 5.0 ± 0.9 points, which indicated a predominance of moderate MGD. Finally, mixed type dry eye disease was found in T2DM patients with palpebral demodicosis, which required treatment for MGD and restoration of the tear film aqueous layer. Acknowledgement: The authors thank O.I. Dragomiretska for her assistance in statistic analysis. References 1.Shokirova MM. [Developing the methodology of comprehensive stage-by-stage treatment of posterior blepharitis with ocular Demodex infestation]. Abstract of Cand Sc (Med) Thesis. Moscow: Fedorov NMRC MNTK Eye Microsurgery. 2017. Russian. 2.Maichuk IuF, Iani EV. [New approaches to the treatment of blepharitis]. Kataraktalnaia I refraktsionnaia khirurgiia. 2012;12(1):59–62. Russian. 3.Gim?nez-G?mez R, Garc?a-Catal?n MR, Gallardo-Galera JM. Tear clearance and ocular symptoms in patients treated with preservative-free prostaglandins. Arch Soc Esp Oftalmol. 2013 Mar;88(3):88-91. 4.Drozhzhina GI. [Inflammatory eyelid disorders]. Odesa: Astroprint; 2011. Russian. 5.Obrubov AS. [Substantiation and efficacy of combination treatment approaches for dry eye syndrome in climacteric women]. Cand Sc (Med) Thesis. Moscow: Pirogov Russian National Research Medical University. 2013. Russian. 6.Auw-Haedrich C, Reinhard T. [Chronic blepharitis: pathogenesis, clinical features, and therapy]. Ophthalmologe. 2007 Sep;104(9):817-26. German. 7.Gumerova EI, Mal’khanov VB, Shevchuk NE. [Results of examination of the local immunity in demodectic blepharoconjunctivitis]. Vestn Oftalmol. Vestn Oftalmol. 2004 Sep-Oct;120(5):16-8. Russian. 8.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92. 9.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1994-2005. 10.Biernat MM, Rusiecka-Zi??kowska J, Pi?tkowska E, Helemejko I, Biernat P, Go?ciniak G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Jpn J Ophthalmol. 2018 Nov;62(6):628-633. 11.Wesolowska M, Knysz B, Reich A, Blazejewska D, Czarnecki M, Gladysz A, et al. Prevalence of Demodex spp. in eyelash follicles in different populations. Arch Med Sci. 2014 May 12;10(2):319-24. 12.Kogan BM, Gorgol VT. [Specificity of Demodex folliculorum and Demodex brevis mites, the causative agents of human Demodicosis]. Ukrainskii zhurnal dermatologii, venerologii, kosmetologii. 2001;21:37-40. Russian. 13.Prozornaia LP. [Diagnosis and treatment of dry eye syndrome in patients with chronic blepharitis]. Abstract of Cand Sc (Med) Thesis. St Petersburg: St Petersburg State Pediatric Medical University. 2014. Russian. 14.Elistratova LL, Nesterov AS, Potaturkina-Nesterova NI. [Current state of the demodicosis problem]. Fundamentalnyie issledovaniia. 2011;(9):67-9. Russian. 15.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1922-9. 16.Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009;579:221-46. 17.G?k?e C, Aycan-Kaya ?, Yula E, ?st?n I, Yengil E, Sefil F, et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res. 2013 Oct;41(5):1752-8. 18.Shamsheer RP, Arunachalam C. A Clinical Study of Meibomian Gland Dysfunction in Patients with Diabetes. Middle East Afr J Ophthalmol. 2015 Oct-Dec;22(4):462-6. 19.Keskin Kurt R, Aycan Kaya O, Karateke A, et al. Increased density of Demodex folliculorum mites in pregnancies with gestational diabetes. Med Princ Pract. 2014; 23:369–72. 20.Kim JH, Chun YS, Kim JC. Clinical and immunological responses in ocular demodicosis. J Korean Med Sci. 2011 Sep;26(9):1231-7. 21.Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797-803. 22.Wang YJ, Ke M, Chen XM. Prospective Study of the Diagnostic Accuracy of the In Vivo Laser Scanning Confocal Microscopy for Ocular Demodicosis. Am J Ophthalmol. 2019 Jul;203:46-52. 23.Pathan R. Prevalence of meibomian gland disease in type II diabetic patients & its clinical presentations. JEBMH. 2015;2(4):356–53. 24.Zhmud TM, Nikolaichuk DV, Nikolaichuk VI. [Improvement of the technique of non-contact portable meibography]. Oftalmologiia. Vostochnaia Evropa. 2018;8(4):488-96. Russian. 25.Yamashita LS, Cariello AJ, Geha NM, Yu MC, Hofling-Lima AL. Demodex folliculorum on the eyelash follicle of diabetic patients. Arq Bras Oftalmol. 2011 Nov-Dec;74(6):422-4. 26.Wise RJ, Sobel RK, Allen RC. Meibography: A review of techniques and technologies. Saudi J Ophthalmol. 2012 Oct;26(4):349-56. 27.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|