J.ophthalmol.(Ukraine).2019;6:15-22.
|
http://doi.org/10.31288/oftalmolzh201961522 Received: 17 September 2019; Published on-line: 31 December 2019 Value of TNF-? (rs1800629) polymorphism in recurrent maculopathy after surgery in a Ukrainian population of T2DM patients Iu.O. Panchenko, Cand Sc (Med) Kyiv Municipal Clinical Hospital “Eye Microsurgery Center”; Kyiv (Ukraine) Laser Plus Medical Center; Lviv (Ukraine) E-mail: panchenko0802@gmail.com Background: Tumor necrosis factor alpha (TNF?) induces insulin resistance in type 2 diabetes mellitus (T2DM). TNF-? -308G/A (rs1800629) polymorphism is associated with T2DM and its complications (particularly, diabetic retinopathy (DR) and diabetic maculopathy (DMP)) since it contributes to TNF-? expression. Purpose: To investigate the value of TNF-? (rs1800629) polymorphism in recurrent DMP after surgery in a Ukrainian population of patients with T2DM. Materials and Methods: The study included 313 patients with T2DM (313 eyes) and diabetic maculopathy. These included patients with mild nonproliferative DR (NPDR; n=40), moderate or severe NPDR (n=92), and proliferative DR (PDR; n=181). Patients received one of the four types of surgical treatment: only three-port closed subtotal vitrectomy (CSTV; n=78); CSTV combined with internal limiting membrane (ILM) peeling (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); and CSTV combined with ILM peeling, PRLC and cataract phacoemulsification (phaco) (n=69). Baseline blood TNF-? levels were measured by enzyme-linked immunosorbent assay and rs1800629 was investigated by polymerase chain reaction. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Results: In patients with DMP, rs1800629 was associated with the development and recurrence of DMP after surgery. Recurrent DMP developed in 96.9% of A/A genotype carriers. Heterozygous G/A genotype carriers had an increased risk (OR = 3.76; 95% CI: 2.27-6.27), whereas homozygous ancestral G/G genotype carriers, a decreased risk (OR = 0.02; 95% CI: 0.01-0.06) for recurrent DMP. TNF-? rs1800629 A-allele carriage was associated with macular edema. Among study patients, central retinal thickness was highest in A-allele carriers, both at baseline and after surgery, and did not decrease in these carriers within the follow-up period. Conclusion: An elevated TNF? blood level in carriers of the minor A-allele of the TNF? rs1800629 polymorphism was a factor promoting recurrent DMP after surgery. Keywords: diabetic maculopathy, type 2 diabetes mellitus, surgical treatment, recurrence, TNF-?, rs1800629
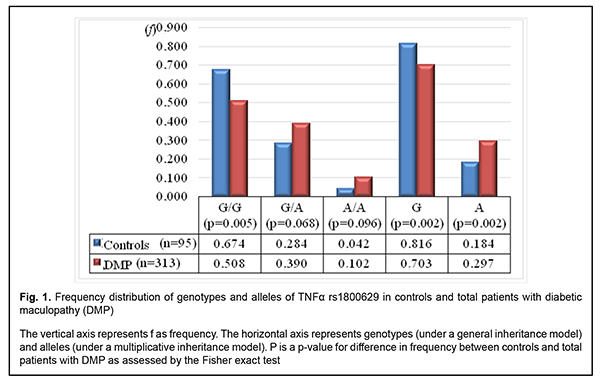
Introduction Ocular complications (particularly, diabetic retinopathy (DR) and diabetic maculopathy (DMP)) are one of the most common complications associated with type 2 diabetes mellitus (T2DM) [1-3]. In addition, DMP develops not in any patient with DR, but DMP incidence increases with an increase in severity of DR. Thus, DMP develops in 3-38% of patients with non-proliferative DR (NPDR), 20-63% of those with pre-proliferative DR, and above 70%% of those with proliferative DR (PDR). It has been reported that pro-inflammatory cytokines, particularly, tumor necrosis factor alpha (TNF?), are important for the development of insulin resistance [4]. The role of TNF? in signaling from the insulin receptor (specifically, inhibiting insulin receptor substrate (IRS)-1 and tyrosine phosphorylation of the insulin receptor and thus suppressing further activation of PI3K/Akt and ERK/MAP kinase pathways and uptake of glucose) constitutes the molecular basis for this effect [5, 6]. Therefore, TNF?-induced IRS-1 inactivation is a mechanism for the development of insulin resistance [7]. In addition, three novel proteins (RIPK1, BIRC2 and BIRC3) have been identified through computational analysis of TNF? protein network, and network analysis showed the interconnectivity between signaling pathways such as TNF receptor, NFkB, Toll-like receptor and apoptosis; these proteins also effect the development of insulin resistance [8]. Genetic factors, particularly, gene polymorphisms, contribute to the development of T2DM and its complications [8-13]. A meta-analysis was applied to integrate the findings from 10 studies (with a total of 1425 T2DM patients and 1116 healthy control subjects) to provide an overall assessment whether TNF-? -308G>A (rs1800629) polymorphism is associated with T2DM risk in a population of Han Chinese subjects. The pooled ORs (95% CIs) for TNF-? -308G>A of A vs. G allele and G/A+A/A vs. G/G genotype were 1.63 (1.17–2.25) and 1.47 (1.17–1.85), respectively [9]. A Brazilian study [10] involving 745 outpatients with T2DM, including 331 subjects without DR, 246 with NPDR, and 168 with PDR, demonstrated that the A allele of the –308G>A polymorphism was (a) more frequent in subjects with PDR than in those with no DR (18.1% vs. 11.5%, corrected P = 0.035), and (b) independently associated with an increased risk of PDR, under a dominant model (adjusted odds ratio [aOR], 1.82; 95% con?dence interval [CI], 1.11–2.98). Indian studies [11, 12] showed the association of TNF-? -308G/A (rs1800629) with the development of T2DM and its complications, which was accompanied by increased blood levels of TNF?, IL-6 and SDF-1. A systemic overview by Luna et al [13] noted contradictory results of prior studies aimed at establishing associations between TNF-? -308G/A (rs1800629) and T2DM in various populations. They believe that ethnic differences may play a role in these conflicting results; the results of these studies suggest the need for further investigation. Therefore, the data above pointed to an association of TNF-? -308G/A (rs1800629) with T2DM and its complications (particularly, DR and DMP), and provided evidence on the need for investigating a possible association of this SNP with recurrent DMP after surgery in a Ukrainian population of patients with T2DM. The purpose of this study was to investigate the value of TNF-? -308G/A (rs1800629) in recurrent DMP after surgery in a Ukrainian population of patients with T2DM. Material and methods The study included 313 patients with DM2 (313 eyes) and diabetic maculopathy. These included patients with mild NPDR (Group 1; n=40), moderate or severe NPDR (Group 2; n=92), and PDR (Group 3; n=181). Ninety-five age- and gender- matched individuals without ocular disorders were used as controls. The patients were ethnic Ukrainians and were not relatives. Each patient underwent an eye examination which included visual acuity assessment, static Humphrey perimetry, refractometry, slit lamp biomicroscopy, gonioscopy, ophthalmoscopy with Volk Super Field lens and Goldmann three-mirror lens (Volk Optical, Mentor, OH) and fundus photography (the ETDRS seven standard fields) and fluorescent angiography with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). In addition, they underwent spectral domain optical coherence tomography (SD-OCT; Copernicus REVO, Optopol Technology Sp, zo.o, Zawiercie, Poland; scan programs, Retina 3D and Retina Raster) and OCT (Retina Angio mode, 6 x 6 mm). Severity of DR and DMP was graded as per the 2002 guidelines of the American Academy of Ophthalmology [3]. Indications for surgical intervention were progressive vision loss; visual field defects in the central and paracentral regions; changes in the quality of vision in the presence of (a) NPDR with refractive macular edema or macular edema with the presence of tangential tractions resulting from incomplete detachment of the posterior hyaloid membrane of the vitreous, (b) PDR with refractive macular edema, presence of epiretinal membranes or presence of tangential or axial retinal tractions and imminent tractional retinal detachment, (c) presence of vitreous, preretinal and/or subhyaloid hemorrhage [14]. Patients received one of the four types of surgical treatment: only three-port closed subtotal vitrectomy (CSTV; n=78); CSTV combined with internal limiting membrane (ILM) peeling (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); and CSTV combined with ILM peeling, PRLC and cataract phacoemulsification (n=69). Follow-up visits were at months 1, 3, 6 and 12 after surgery. Enzyme-linked immunosorbent assay (ELISA) was used to measure blood TNF? levels with kits from Bender Medsystems (Austria). The intensity of staining of the enzymatic reaction product was measured quantitatively with a Multiskan Ex plate reader (ThermoFisher Scientific, Vantaa, Finland). TNF-? -308G/A (rs1800629; locus, 6p21.33) polymorphism was investigated by real-time polymerase chain reaction (PCR) using TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA; context sequence, GAGGCAATAGGTTTTGAGGGGCATG[A/G]GGACGGGGTTCAGCCTCCAGGGTCC ) and Gene Amp® 7500 PCR System (Applied Biosystems, Foster City, CA). At the first phase of the study, DNA extraction from venous blood was done using the Invitrogen™ PureLink Genomic DNA kit for purification of genomic DNA (Invitrogen Inc.) following the manufacturer’s instructions. The study design and protocol were approved by the ethics committee of Shupik National Medical Academy of Postgraduate Education and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. The percentage of patients with recurrent DMP after different surgical treatments, and the relationship of recurrent DMP with TNF-? -308G/A (rs1800629) were determined. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Since the Kolmogorov-Smirnov, Andersen–Darling and squared chi tests found that distributions of variational series data were not normal (p < 0.05), the quantitative data were presented as median, first quartile (Q1) and third quartile (Q3) values of variational series. Kruskal-Wallis (H) and Mann-Whitney (U) tests were applied for comparisons of independent samples. Contingency tables were made and non-parametric Pearson-Yates chi-square test was applied for comparison of categorical variables. The level of significance p ? 0.05 was assumed. Results In the first phase of the study, frequency distributions of genotypes and alleles were compared between the cases of diabetic maculopathy and controls (Fig. 1).
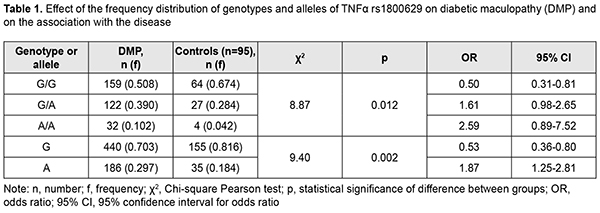
The homozygous ancestral (G/G) genotype for rs1800629 was 1.3-fold and significantly (p = 0.005) less frequent in patients than in controls. In addition, the heterozygous G/A genotype and minor A/A genotype were 1.4-fold and 2.4-fold, respectively, and not significantly (р = 0.068 and р = 0.096, respectively) more frequent in patients than in controls. Moreover, the ancestral (G) allele was found 1.16-fold and significantly (р=0.002) less frequently, whereas the minor (A) allele was found 1.61-fold and significantly (р=0.002) more frequently in patients than in controls. Therefore, there were significant differences in frequency distributions of the G/G genotype and both alleles between patients and controls. Based on these findings, we assessed the effect of the genotype and allele frequency distributions on the development of DMP, and the degree of their association with the disease (Table 1). Hardy-Weinberg equilibrium was met in the controls and cases (?2=0.28; df=1; p=0.597 and ?2=1.40; df=1; p=0.237, respectively).
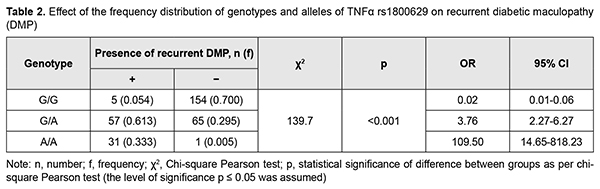
Analysis of the effect of genotype distribution based on a general inheritance model (with a 3 x 3 contingency table) showed an association between this distribution and the occurrence of DMP (?2=8.87; p=0.0012). The carriers of the homozygous minor A/A genotype at rs1800629 had a 2.6-fold (OR = 2.59; 95% CI: 0.89-7.52) higher risk of developing DMP. The heterozygous G/A genotype was also found to increase the risk of developing DMP (OR = 1.61; 95% CI: 0. 98-2.65). Therefore, both the wild and the heterozygous genotypes of rs1800629 were found to be DMP-risk genotypes. Since the homozygous G/G genotype at rs759853 was associated with a twofold decreased risk (OR = 0.50; 95% CI: 0.31-0.81) for DMP, it can be considered as protective in our population. Analysis of the effect of allele distribution based on a multiplicative inheritance model (with a 2 x 2 contingency table) showed an association between this distribution and the occurrence of DMP (?2=9.40; p=0.002). The minor A allele was associated with a 1.9-fold increased risk (OR=1.87; 95% CI: 1.25-2.81), whereas the ancestral G allele was associated with a 1.9-fold decreased risk (OR=0.53; 95% CI: 0.36-0.80) for the disease. The comparison of dominant and recessive models of inheritance showed that the genotype distribution under a dominant model (G/G versus G/А+А/А) was statistically significant by Pearson chi-square test (?2=8,05; P=0.004), which confirmed an association between rs1800629 and DMP in the presence of the minor A allele (for G/А+А/А, OR=2.0; 95% CI: 1.23-3.24); these results are in agreement with reports from others [9, 10]. In the current study, we found no significant differences in genotype or allele frequencies between patients operated with different CSTV techniques, which was evidence of genetic homogeneity of patients before surgery (p > 0.05). In addition, we found no significant differences in genotype or allele frequencies (p = 0.975 and p = 0.870, respectively) between groups of patients, whereas previous studies by others [4, 9, 10] found that frequencies of minor A/A genotype and A allele were significantly higher in patients with PDR. At first glance, our latter finding might seem paradoxical. It should be, however, taken into consideration that, in the current study, patients from all groups had indications (like progressive vision loss; visual field defects in the central and paracentral regions; changes in the quality of vision; refractive macular edema; or presence of vitreous, preretinal and/or subhyaloid hemorrhage) for surgery. Therefore, all our patients, irrespective of the stage of diabetic retinopathy, did have a marked pathological process, likely due to the presence of unfavorable factors, particularly, those of genetic origin. This was indirectly evidenced by the distribution of patients among groups, with the numbers and percentages of patients in the moderate or severe NPDR group (group 2: 92 patients; 29.4%) and PDR group (group 3: 181 patients; 57.8%) being substantially higher than in the mild NPDR group (group 1: 40 patients; 12.8%). In other words, the current study included patients exhibiting a rapid DMP progression and requiring surgery, which resulted in forced selection of DR variants with a very poor clinical course. We believe that this feature explains also the high overall incidence and percentage of recurrent DMP (93 eyes; 29.7%) within 12 months after surgery, and the absence of significant difference (p = 0.18) in percentage of recurrent DMP between groups (group 1: 27.5%, 11 eyes; group 2: 22.8%, 21 eyes; and group 3: 33.7%, 61 eyes). This led to the main objective of this study, to examine the relationship between the TNF-? (rs1800629) SNP and recurrent DMP after surgery (Table 2). The ancestral (G/G) genotype was found significantly (p < 0.001) less frequently in patients with recurrent DMP than in those without it. However, frequencies of risk variants of the TNF-? (rs1800629) SNP (heterozygotes G/A or minor homozygotes A/A) were significantly (p < 0.001) increased in the former patients.
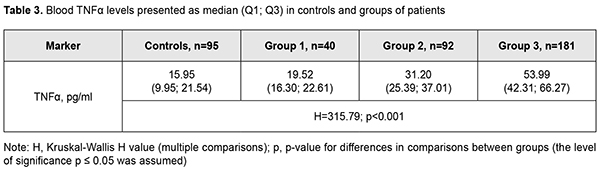
It was of interest that, among the study cohort, there were 32 (10.2%; Table 1) minor A/A genotype carriers, and almost all of them (31; 96.9%) had recurrent DMP after surgery. This directly pointed to the decisive importance of the minor A/A genotype at the TNF-? (rs1800629) SNP for the development of recurrent DMP. The heterozygous G/A genotype at rs759853 was associated with a 3.8-fold increased risk (OR = 3.76; 95% CI: 2.27-6.27), whereas the protective homozygous ancestral G/G genotype was associated with a 50-fold decreased risk (OR = 0.02; 95% CI: 0.01-0.06) for recurrent DMP. Further analysis of genotype frequencies at different follow-up time points revealed the same tendency, a.i., at each time point, frequencies of risk variants of the TNF-? (rs1800629) SNP were significantly (p < 0.001) higher in patients with recurrent DMP than in those without it. Therefore, our findings directly demonstrated an important role of the TNF-? (rs1800629) SNP in recurrent DMP after surgery, irrespective of DR severity, surgical technique utilized for CSTV, and follow-up time points. It has been reported that elevated blood TNF? levels in patients with T2DM corresponded to the severity of the disease and its complications, particularly, DR [11]. In the current study, elevated baseline blood cytokine levels corresponded to progression in DR stage (Table 3), and these levels in groups 1, 2 and 3 were found to be 1.2-fold (р=0.005), 2.0-fold and 3.4-fold (р < 0.001) increased compared to controls. Patients from group 3 (PDR) and those from group 1 (mild NPDR) exhibited maximum and minimum blood TNF? levels, respectively.
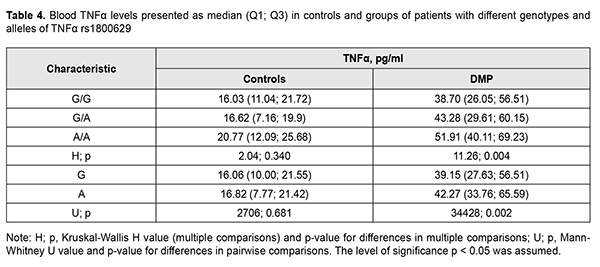
Table 4 presents median blood TNF? levels in our patients with different genotypes of rs1800629.
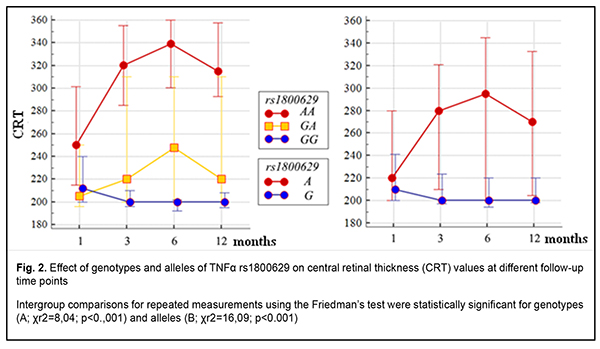
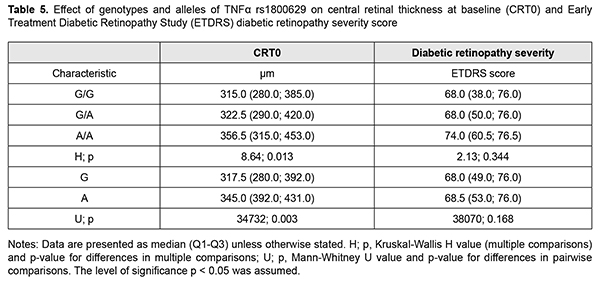
It is noteworthy that median blood TNF? levels were 2.4-fold to 2.6-fold higher in patients with any genotype of rs1800629 than in controls (р < 0.001). In addition, blood TNF? levels in carriers of risk A/A genotype and A allele were higher than in carriers of other genotypes; this was true both for patients with DMP and controls, but the difference was statistically significant only for patients. Moreover, differences in these levels for patients with different genotypes and for those with different alleles of rs1800629 were also significant (p = 0.004 and р=0.002, respectively). In addition to vascular manifestations, diabetic macular edema (DME) is of importance in patients with DMP. Previously, we have found it in eyes with central retinal thickness (CRT) of more than 250 ?m [15]. It was found that DME frequency in recurrent DMP increased with an increase in time between surgery and follow-up and did not depend on surgical technique. At 1 month, 3 months and 12 months after surgery, DME was found in 30.4%-53.3%, 73.3%-86.7%, and 68.4%-100% of eyes with recurrent DMP. Preoperative CRT value varied from 195 ?m to 880 ?m (median value, 358.09±6.96 ?m). There was no statistically significant difference in preoperative CRT between groups; this is in agreement with the idea of a decisive value of preoperative clinically significant DME that affected visual acuity and was an indication for surgery [3]. Postoperatively, in all groups, CRT decreased by 113-118 ?m and became normal (р < 0.001). No significant difference in CRT between groups was observed at any follow-up time point (p > 0.2). Identifying a relationship of the TNF-? (rs1800629) SNP with baseline CRT (CRT0) and severity level of DR (ETDRS scale) was also of some interest (Table 5) [16].
CRT0 values were higher (a) in heterozygous G/A or minor homozygous A/A carriers than in ancestral homozygous G/G carriers (р=0.013) and (b) in A allele carriers than in ancestral G allele carriers (р = 0.003). There was no significant difference in ETDRS severity score under conditions of such distribution (p > 0.1). Therefore, among patients with recurrent DMP, carriers of the A allele of the TNF-? (rs1800629) SNP had a greater CRT before surgery. In addition, minor homozygous A/A carriers exhibited a greater CRT (Fig. 2A) at any follow-up time point (р < 0.001). Moreover, such patients showed a somewhat gradual increase in median CRT at subsequent follow-up visits after month 1 (р < 0.001). Furthermore, CRT was 1.1-fold to 1.5-fold greater in minor A allele carriers than in ancestral G allele carriers at any follow-up time point (р < 0.001). Therefore, among the patients treated for DMP, CRT was greater in the risk A/A genotype carriers and risk A allele carriers, and did not decrease in these risk patients within the follow-up period. This confirmed the pathogenetic role of the TNF-? (rs1800629) SNP in the development of DME in recurrent DMP after surgery. A series of univariate logistic regression models were built to prove the impact of the TNF-? (rs1800629) SNP on recurrent DMP, development of DME, TNF-? levels, CRT, and, correspondingly, on severity of DR (ETDRS scale). The model analysis demonstrated that the TNF-? (rs1800629) SNP exerted a significant effect on recurrent DMP (р < 0.001) and development of DME (р ? 0.001) at all follow-up time points, and a significant effect on an increase in TNF-? blood levels (р = 0.002) and on CRT at baseline (р = 0.008), at month 1 (р = 0.006) and at subsequent follow-up time points (р < 0.001). Therefore, the results of the model analysis proved the impact of the SNP on recurrent DMP after surgery. Discussion Dozens of studies reporting on identification of associations of SNPs of proinflammatory cytokine genes (TNF?, IL-6 and IL-1?) with T2DM and its complications have been subjected to a meta-analysis [17]. Individual SNP analysis showed the highest association of TNF-? rs1800629-A/A genotype (OR?=?2.75, 95% CI?=?1.64-4.59, P?=?0.001) with T2DM and diabetic nephropathy [18]. Those authors related it to the fact that TNF-? expression was increased by more than four-folds (n-fold=4.43±1.11), which was accompanied by a substantial increase in TNF-? blood level in T2DM patients carrying the minor A/A genotype [18]. Another study [19] found that the minor A/A genotype and minor A allele of rs1800629 significantly increased TNF-? blood level, which was more expressed in patients with complications of T2DM. Therefore, one can identify the following pattern of pathogenetic relationship: carriage of the minor A allele is a pathogenetic factor for T2DM and its complications, with TNF-? overexpression leading to excessive TNF-? production. With regard to our findings, it can be noted that TNF-? (rs1800629) polymorphism was associated not only with the development of DMP in T2DM, but also with the development of recurrent DMP after surgery. This could be explained by elevated blood TNF-? levels in A allele carriers. Conclusion First, distributions of genotypes and alleles of the TNF? rs1800629 were associated with the development of the disease (р=0.012 and р=0.002, respectively) in a Ukrainian cohort of patients with DR, DMP and T2M. Carriers of genotypes with the minor A allele had a 2.0-fold higher risk of developing DMP (under a dominant model, OR = 1.82; 95% CI, 1.23–3.24’ p = 0.004). Second, the minor A/A genotype at rs1800629 determined the development of recurrent DMP, and 96.9% of its carriers exhibited recurrent DMP in the postoperative period. The heterozygous G/A genotype at rs759853 was associated with an increased risk (OR = 3.76; 95% CI: 2.27-6.27), whereas the homozygous ancestral G/G genotype, with a decreased risk (OR = 0.02; 95% CI: 0.01-0.06) for recurrent DMP. Recurrent DMP frequency did not depend on DR stage, surgical technology or follow-up time point. Third, carriage of the minor A allele of the TNF? rs1800629 polymorphism was associated with a greater CRT both at baseline and within 12 months after surgery. CRT was 1.1-fold to 1.5-fold greater in minor A allele carriers than in ancestral G allele carriers at any follow-up time point (р < 0.001), and did not decrease in A-allele carriers within the follow-up period. Finally, our findings and analysis of recent literature allow us to state that an elevated TNF? blood level in carriers of the minor A-allele of the TNF? rs1800629 polymorphism was a pathogenetic factor promoting recurrent DMP after surgery. References 1.Pankiv VI. [Workshop No. 162. Diabetes mellitus: Diagnostic criteria, etiology and pathogenesis]. Mizhnarodnyi endokrynologichnyi zhurnal. 2013;8:53-64. Ukrainian. 2.Type 2 Diabetes Complications. Web-resource: https://www. endocrineweb.com/conditions/type-2-diabetes/type-2-diabetes-complications 3.Balashevich LI, Izmailov AS. [Diabetic ophthalmopathy]. St Petersburg: Chelovek; 2012. Russian. 4.Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-? and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016 Oct;86:100-9. 5.IRS1 – Insulin receptor substrate 1 – Homo sapiens (Human) – IRS1 gene & protein". www.uniprot.org. Retrieved 2016-04-21. 6.Takaguri A. [Elucidation of a new mechanism of onset of insulin resistance: effects of statins and tumor necrosis factor-? on insulin signal transduction]. Yakugaku Zasshi. 2018;138(11):1329-1334. Japanese. 7.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565-82. 8.Jamil K, Jayaraman A, Ahmad J, Joshi S, Yerra SK. TNF-alpha -308G/A and -238G/A polymorphisms and its protein network associated with type 2 diabetes mellitus. Saudi J Biol Sci . 2017 Sep;24(6):1195-1203. 9.Liu ZH, Ding YL, Xiu LC, Pan HY, Liang Y, Zhong SQ, Liu WW, Rao SQ, Kong DL. A meta-analysis of the association between TNF-? -308G>A polymorphism and type 2 diabetes mellitus in Han Chinese population. PLoS One. 2013;8(3):e59421. 10.Sesti LF, Crispim D, Canani LH, Polina ER, Rheinheimer J, Carvalho PS, Gross JL, Santos KG. The -308G>A polymorphism of the TNF gene is associated with proliferative diabetic retinopathy in Caucasian Brazilians with type 2 diabetes. Invest Ophthalmol Vis Sci. 2015 Jan 29;56(2):1184-90. 11.Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-? and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015 Jul 1;565(1):62-7. 12.Doody NE, Dowejko MM, Akam EC, Cox NJ, Bhatti JS, Singh P, Mastana SS. The role of TLR4, TNF-? and IL-1? in type 2 diabetes mellitus development within a North Indian population. Ann Hum Genet. 2017 Jul;81(4):141-146. 13.Luna GI, da Silva IC, Sanchez MN. Association between -308G/A TNFA Polymorphism and Susceptibility to Type 2 Diabetes Mellitus: A Systematic Review. J Diabetes Res. 2016;2016:6309484. 14.Mogilevskyy SIu, Panchenko IuA. [Features of diabetic maculopathy in patients with type 2 diabetes mellitus]. Arkhiv oftalmologii Ukrainy. 2018;6(2 (11)): 28-32. Russian. 15.Mogilevskyy SIu, Panchenko IuO, Ziablytsev SV. Predicting the risk of diabetic retinopathy-assosiated macular edema in patients with type 2 diabetes mellitus. Journal of Ophthalmology (Ukraine). 2019;3(488):3-8. 16.Early Treatment Diabetic Retinopathy Study Research design and baseline patient characteristics. ETDRS, report number 7. Ophthalmology. 1991; 98(5) Suppl.:741-56. 17.Zhao Y, Li Z, Zhang L, Zhang Y, Yang Y, Tang Y, Fu P. The TNF-alpha -308G/A polymorphism is associated with type 2 diabetes mellitus: an updated meta-analysis. Mol Biol Rep. 2014 Jan;41(1):73-83. 18.Hameed I, Masoodi SR, Malik PA, Mir SA, Ghazanfar K, Ganai BA. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018 Jun 30;661:51-59. 19.Umapathy D, Krishnamoorthy E, Mariappanadar V, Viswanathan V, Ramkumar KM. Increased levels of circulating (TNF-?) is associated with (-308G/A) promoter polymorphism of TNF-? gene in Diabetic Nephropathy. Int J Biol Macromol. 2018 Feb;107(Pt B):2113-2121.
Conflict of Interest: The authors declare no conflicts of interest with regard to this paper.
|