J.ophthalmol.(Ukraine).2019;6:63-69.
|
http://doi.org/10.31288/oftalmolzh201966369 Received: 25 November 2019; Published on-line: 06 January 2020 SS-OCT-derived morphometric changes in the choroid in patients with age-related macular degeneration D.O. Peretiahina, Post-graduate Student; N.A. Ulianova, Dr Sc (Med), Prof Odesa National Medical University; Odesa (Ukraine) E-mail: d.peretyagina@gmail.com TO CITE THIS ARTICLE: Peretiahina DO, Ulianova NA. SS-OCT-derived morphometric changes in the choroid in patients with age-related macular degeneration. J.ophthalmol.(Ukraine).2019;6:63-69. http://doi.org/10.31288/oftalmolzh201966369
Background: Since the prevalence of late age-related macular degeneration (AMD) is increasing, investigating the role of the choroid in the pathogenesis of the disease is particularly important. Purpose: To examine swept-source optical coherence tomography (SS-OCT)-derived subfoveal choroidal thickness (SFCT) in patients differing in phenotypic manifestations of AMD. Material and Methods: Fifty-five AMD patients (67 eyes; age, 64 to 75 years) and 8 comparably matched-aged healthy controls (13 eyes) underwent SS-OCT measurement of SFCT. They were divided into 6 groups: healthy individuals without signs of AMD and patients with drusen; type 1 choroidal neovascularization (CNV); serous retinal pigment epithelium (RPE) detachment; type 1 CNV; and geographic atrophy (GA). Statistical analyses were conducted using Statistica software, version 13.5.0.17. Both parametric and non-parametric techniques were used. Results: Mean SFCT in patients with GA was 47.8% lower than in controls, 45.7% lower than in patients with drusen, and 51.7% lower than in patients with type 1 CNV (p < 0.05). We found no significant difference in mean SFCT between patients with GA and those with RPE detachment, or between the former and those with type 2 CNV. Patients with AMD and age-matched healthy controls were divided into four clusters based on choroidal thickness (cluster I, 105 ± 4.8 ?m; cluster II, 188 ± 3.5 ?m; cluster III, 266 ± 4.6 ?m; and cluster IV, 408 ± 14.9 ?m). There was no difference in cluster distribution between eyes with type 1 CNV and normal age-matched eyes. Of the eyes with drusen, most (81.8%) were from cluster II. A statistically significant majority of eyes with serous RPE detachment, type 2 CNV, and dry AMD were assigned to clusters I and II (p < 0.05). Conclusion: Patient groups differing in phenotypic manifestations of AMD were found to differ in the mean magnitude of SS-OCT-derived SFCT, and could be classified into 4 clusters on the basis of their value of SFCT. Keywords: subfoveal choroidal thickness, age-related macular degeneration; optical coherence tomography
Introduction A recent review and meta-analysis have reported that approximately 67 million people in the EU are currently affected by any age-related macular degeneration (AMD) and this number is expected to increase by 15% until 2050 [1]. Since a similar tendency of increasing prevalence of AMD can be observed in Ukraine, investigating the pathogenesis of changes in the neurosensory retina is important for optimizing monitoring and management of the macular disorder. AMD is known to be a multifactorial disease that is characterized by stages of progression [2]. Although a progressive loss of vision occurs in AMD as a result of injury to the macular photoreceptors, there are various phenotypic manifestations of this pathological process [3, 4]. Particularly, Saade and co-authors [5] note that the pathogenesis of loss of retinal pigment epithelium (RPE) and neuroepithelium in late dry AMD (geographic atrophy) and that in neovascular AMD are underlain by different primary irregularities in retinal vascular plexuses and choroidal vessels [5]. Although a large portion of current studies on the pathogenesis of AMD have investigated the role of drusen and changes in the RPE, and choroidal changes during AMD progression are still a subject of discussion, there is a concept that it is pathological changes in perfusion of retinal and choroidal vasculature that are early predictors of the development of AMD [6-9]. The concept has been based on findings of ultrasound studies and was further developed with advances in enhanced depth imaging spectral-domain optical coherence tomography (OCT) and recent use of ‘‘en face’’ OCT allowing a cross-sectional, noninvasive, layer-by-layer visualized reconstructed image of the involved retina and choroid in vivo [4]. There have been a number of OCT studies on alterations in choroidal structure under conditions of physiological aging or pathological changes in the macula [10, 11]. Of importance are studies on not only choroidal thickness, but also on assessment of rheoophthalmographic indices [12]. However, no uniform normative database is available, and various scanning protocols are used for choroidal thickness measurements in different topographic sites. In addition, the choroid is a highly vascular structure whose thickness varies with age, gender, refractive error, intraocular pressure, perfusion pressure, diurnal rhythms, etc [13]. Therefore, investigating choroidal structure and identifying differences in the state of the choroid between different stages of the pathological process will bring us nearer to identifying the role of the choroid in the progression and clinical course of AMD. The purpose of the study was to examine swept-source (SS)-OCT subfoveal choroidal thickness (SFCT) in patients differing in phenotypic manifestations of AMD. Material and Methods This was an open, prospective, non-interventional comparison study. This study followed the ethical standards stated in the Declaration of Helsinki and informed consent was obtained from all individuals enrolled in the study. Fifty-five AMD patients (67 eyes; age, 64 to 75 years) and 8 comparably matched-aged healthy controls (13 eyes) were under observation at the Eye Medical Center of the Odesa National Medical University. The inclusion criterion was no history of intravitreal anti-vascular endothelial growth factor (VEGF) treatment for AMD. Exclusion criteria were glaucoma, past history of phacoemulsification cataract surgery, retinal pigment epithelium (RPE) detachment exceeding 500 ?m, high ametropia (> 3D), or axial myopia. Patients were divided into groups (Table 1) depending on phenotypic manifestations of AMD which were identified by fundoscopy, fluorescein angiography and OCTA.
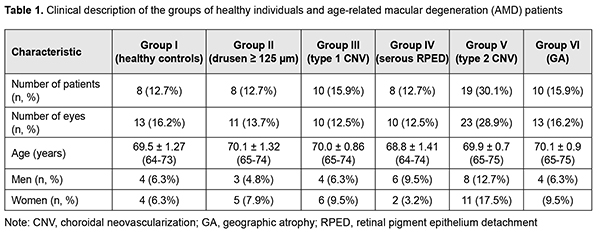
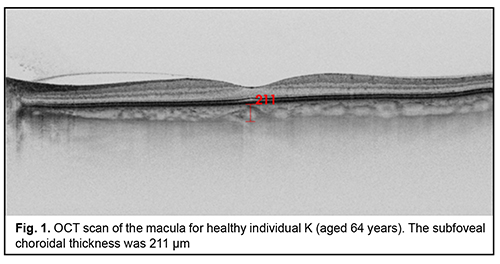
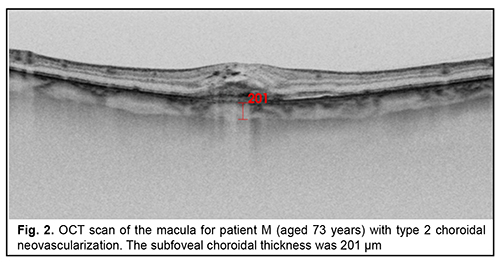
Patients were categorized by the clinical fundus picture. A high-definition Triton Plus® SS-OCT system (Topcon Corporation, Tokyo, Japan) was used for morphometric assessment of the choroid. SS-OCT uses a wavelength of 1050 nm, compared with 840 nm in SD-OCT, to allow for the less reflection and absorption of photoreceptors and RPE cells, scanning a broader field, and improved depth penetration and thus sharper choroidal imaging. In addition to measuring choroidal thickness, SS-OCT enables further analysis of the different choroidal layers that were difficult to distinguish in SD-OCT scans. A Radial scan pattern (1024?12, 9 mm) was used to measure the SFCT. Measurements were performed at the same time of the day. The SFCT was defined as the distance between the Bruchs membrane and the inner scleral surface at the central fovea. SFCT measurements were acquired by the in-built caliper tool of the integrated SS-OCT software (ImageNet). Negative images were used to allow for better visualization of the choroid. Choroidal borders were identified automatically and adjusted in accordance with the location of the sclerochoroidal interface, particularly, if the choroidal thickness was more than 300 ?m. Figures 1 to 3 present examples of SFCT measurements in patients of the groups.
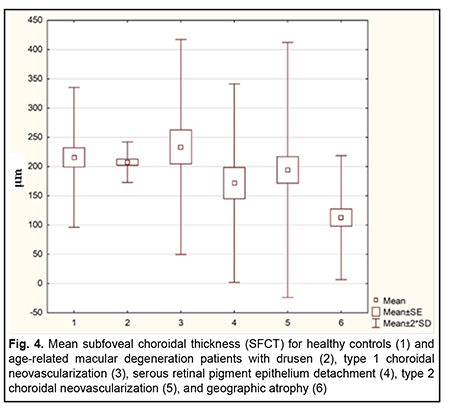
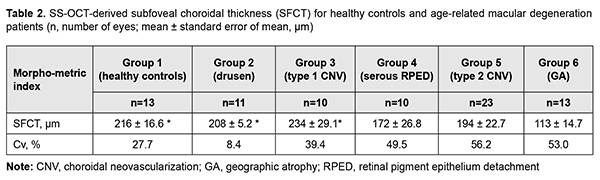
Statistical analyses were conducted using Statistica (StatSoft, Tulsa, OK, USA) software, version 13.5.0.17. Both parametric and non-parametric techniques were used. Mean, standard deviation (SD) and variation coefficient (Cv) were calculated. Individual group data were tested for normality using the Shapiro–Wilk test. Differences among groups were compared using the Kruskal-Wallis test. A cluster analysis was used to group patients according to the choroidal thickness. After the clusters were formed, the next step was to calculate mean SFCT values using descriptive statistics. Differences among clusters were evaluated using analysis of variance. A Newman Keuls test was used to identify any differences between the groups if the null-hypothesis was rejected. Results and Discussion There was a difference in SFCT values among patients differing in retinal changes in AMD (Table 2). Patients with dry AMD had the lowest mean values of SFCT. Mean SFCT in patients with dry AMD was 47.8% lower than in controls, 45.7% lower than in patients with drusen, and 51.7% lower than in patients with type 1 choroidal neovascularization (CNV). We found no significant difference in mean SFCT between patients with dry AMD and those with RPE detachment, or between the former and those with type 2 CNV. In addition, no significant differences were found among healthy controls, patients with type 1 CNV, type 2 CNV, dry AMD, and RPE detachment. There was, however, variability in SFCT in all groups excepting that of patients with drusen (Fig. 4). The data obtained suggested that there is a variety of phenotypic manifestations of changes in choroidal choriocapillaries in different clinical stages of AMD.
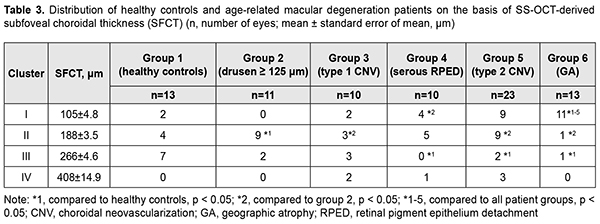
Cluster analysis found that AMD patients could be classified into 4 clusters on the basis of their value of SFCT, with a statistically significant difference in SFCT between patients of different clusters (p < 0.01). Mean SFCT for clusters I, II, III and IV were 104 ± 5.3 ?m, 188 ± 4.0 ?m, 257 ± 5.1 ?m, and 408 ± 16.4 ?m, respectively (Fig. 5). We also analyzed the percentage of eyes from each group in each SFCT cluster (Table 3). In healthy controls, SFCT cluster III was the most common (53.8%), followed by cluster II (30.8%) and cluster I (15.4%). The percentage of eyes from SFCT cluster II for patients with drusen was (81.8%) significantly larger than for healthy controls (p = 0.013). In patients with RPE detachment, 90% of eyes were assigned to clusters I and II, and no eyes were assigned to cluster III, which was significantly different from the distribution seen in healthy controls (p = 0.006). No significant difference in cluster distribution was observed between patients with type 1 CNV and healthy controls. In patients with type 2 CNV, 39.1% and 39.1% of eyes were assigned to clusters 1 and 2, respectively, and the minimum percentage of eyes was also assigned to cluster III (8.7%), which was significantly different from the distribution seen in healthy controls (p = 0.003). In patients with dry AMD, 84.6% of eyes were assigned to cluster I, which was significantly different from distributions seen in other groups (р < 0.05).
Our findings for choroidal morphometric characteristics in patients with AMD are in general agreement with those reported by others. Morphological changes in the choroid with the transition from early to late AMD are important, and are commonly accompanied by the development of choroidal neovascularization; the latter is, however, not observed in late dry AMD. It is known that capillaries have a segmental distribution in the choroid, and the vascular lobule is a structural and functional unit of the choroid; however, there are not lobules, but mostly peripapillary arterioles with numerous anastomoses in the peripapillary choroid and the choroid beneath the macula [15]. Some atrophy of choriocapillaries in the choroid beneath the macula is common with age, but even in eyes with early AMD, in the absence of RPE loss, choriocapillaris atrophy indices were significantly higher compared to age-matched normal eyes [16]. Seddon and colleagues [17] used confocal microscopy to examine histopathological changes in AMD, and found that neovascular buds were adjacent to areas of choriocapillaris loss [17]. We believe that this might be explained by an increase in VEGF in response to hypoxia in the choroid, which should be directed at re-vascularization of ischemic areas. In some cases, however, progression of atrophic changes in choriocapillaris occurs without neovascularization. Thus, a critical reduction in SFCT is observed in geographic atrophy or late, dry AMD, which is the most unfavorable condition with regard to chances for improvements in visual acuity. In the current study, the mean SFCT was statistically significantly less in patients with geographic atrophy than in other groups. That is, such a condition can be seen as a consequence of inefficient adaptive response aimed at protecting the neurosensory retina in ischemic and hypoxic conditions in patients of this age group. In the opinion of Biesemeier et al [18], in AMD, the initial pathological changes are initiated by choroidal atrophy that develops with age and leads to death of RPE cells and photoreceptors; the areas of reduced perfusion were found to significantly exceed the areas of foci of RPE loss and injury to photoreceptors in geographic atrophy, allowing for a conclusion that changes in choriocapillaris precede those in RPE cells and photoreceptors [18]. Lengyel et al [19], however, believe that it is RPE dysfunction (i.e., drusen or RPE detachment) that results in choriocapillaris impairments (particularly, mechanical compression of choriocapillaris) with reduced choriocapillaris perfusion, and with time leads to apoptosis of RPE cells and photoreceptors. We found no significant difference in SFCT between patients with drusen or RPE detachment and age-matched normal individuals, which indicates that RPE dysfunction has no significant effect on SFCT. Studies on interrelationship of SFCT with RPE detachment height or drusen size might allow for identifying these changes. In spite of a lack of agreement between the above opinions, changes in the choroid (especially a reduction in choroidal thickness) have a key role in dry AMD and will inevitably result in impaired blood supply and elimination of metabolic products in the outer retinal layers. Recent advances in anti-VEGF therapy for conditions associated with neovascularization of various origins, particularly, chorioretinitis [20], increasing attention is given to morphometric studies of the choroid in patients with choroidal neovascularization. In healthy individuals and patients with dry AMD, a strong correlation was found between choroidal thickness and age, and a mild correlation between these parameters was found in patients with active wet AMD. Particularly, in a study by Spraul et al [21], the submacular choriocapillaris density was higher in eyes with AMD than in eyes without AMD. In the current study, there was no significant difference in SFCT between patients with neovascular AMD and healthy controls, and the SFCT was greater in patients with type 1 CNV than in those with dry AMD. The cluster analysis allowed for identifying certain distinctions among patients with type 1 CNV and type 2 CNV. Thus, among patients with type 1 CNV, the SFCT cluster distribution was almost comparable with that for healthy controls, whereas among patients with type 2 CNV, most had lower SFCT values. In our opinion, these findings demonstrate an adaptive response of the choroid to hypoxia in the outer retinal layers, which occurs in transformation of the RPE and Bruch’s membrane in the course of AMD progression, especially in occult subretinal membranes. We believe that the absence of a statistically significant reduction in SFCT in eyes with late neovascular AMD compared to normal age-matched eyes is associated with a possible increase in levels of vasoactive substances and angiogenesis factors, activity of the complement system, and development of CNV. Therefore, our findings of changes in SS-OCT-derived morphometric characteristics of the choroid in patients differing in phenotypic manifestations of AMD stress the role of impaired regional hemodynamics in AMD, and warrant further research on disease course which should take into account the genetically determined distinctive features of each case. Conclusion First, there was a difference in SFCT values among patients differing in phenotypic manifestations of AMD. Mean SFCT in patients with dry AMD was 47.8% lower than in healthy controls, 45.7% lower than in patients with drusen, and 51.7% lower than in patients with type 1 choroidal neovascularization (p < 0.05). There was no significant difference in mean SFCT between patients with dry AMD and patients with RPE detachment, or between the former and those with type 2 choroidal neovascularization. Second, patients with AMD and age-matched healthy controls were divided into four clusters based on choroidal thickness (cluster I, 105 ± 4.8 ?m; cluster II, 188 ± 3.5 ?m; cluster III, 266 ± 4.6 ?m; and cluster IV, 408 ± 14.9 ?m). Compared to mean values, distribution by clusters is more sensitive to differences between patient groups with different phenotypic manifestations of AMD. There was no difference in cluster distribution between eyes with type 1 CNV and normal age-matched eyes. Of the eyes with drusen, most (81.8%) were from cluster II. A statistically significant majority of eyes with serous RPE detachment, type 2 CNV, and dry AMD were assigned to clusters I and II (p < 0.05). References 1.Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol. 2019 Nov;11. 2.Garc?a-Layana A, Cabrera-L?pez F, Garc?a-Arum? J, Arias-Barquet L, Ruiz-Moreno JM. Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging. 2017 Oct 3;12:1579-1587. Doi: 10.2147/CIA.S142685. eCollection 2017. 3.Newman AM, Gallo NB, Hancox LS, Miller NJ, Radeke CM, Maloney MA, Cooper JB, Hageman GS, Anderson DH, Johnson LV, Radeke MJ. Systems levelanalysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012 Feb 24;4(2):16. 4.Lu L, Xu S, He F, Liu Y, Zhang Y, Wang J, Wang Z, Fan X. Assessment of Choroidal Microstructure and Subfoveal Thickness Change in Eyes With Different Stages of Age-Related Macular Degeneration. Medicine (Baltimore). 2016 Mar;95(10):e2967. 5.Saade C, Ganti B, Marmor M, Freund KB, Smith RT. Risk characteristics of the combined geographic atrophy and choroidal neovascularization phenotype in age-related macular degeneration. Br J Ophthalmol. 2014;98:1729-1732. 6.Invernizzi A, Benatti E, Cozzi M, Erba S, Vaishnavi S, Vupparaboina KK, Staurenghi G, Chhablani J, Gillies M, Viola F. Choroidal Structural Changes Correlate With Neovascular Activity in Neovascular Age Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2018 Aug 1;59(10):3836-3841. 7.Kumar JB, Wai KM, Ehlers JP, Singh RP, Rachitskaya AV. Subfoveal choroidal thickness as a prognostic factor in exudative age-related macular degeneration. Br J Ophthalmol. 2019 Jul;103(7):918-921. 8.Ryoo NK, Ahn SJ, Park KH, Ahn J, Seo J, Han JW, Kim KW, Woo SJ. Thickness of retina and choroid in the elderly population and its association with Complement Factor H polymorphism: KLoSHA Eye study. PloS One. 2018 Dec 31;13(12):e0209276. 9.Marybeth K. Farazdaghi, Katayoon B. Ebrahimi. Role of the Choroid in Age-related Macular Degeneration: A Current Review. J Ophthalmic Vis Res. 2019 Jan-Mar; 14(1): 78-87. 10.Esmaeelpour M, Ansari-Shahrezaei S, Glittenberg C, Nemetz S, Kraus MF, Hornegger J, Fujimoto JG, Drexler W, Binder S. Choroid, Haller’s, and Sattler’s layer thickness in intermediate age-related macular degeneration with and without fellow neovascular eyes. Invest Ophthalmol Vis Sci. 2014 Jul 22;55(8):5074-5080. 11.Coscas F, Puche N, Coscas G, Srour M, Fran?ais C, Glacet-Bernard A, Querques G, Souied EH. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Jan 3;55(1):64-69. 12.Korol AR, Khramenko NI, Zadorozhnyy OS, Kustrin TB. [Relationship of morphometric parameters of the vascular membrane and blood supply of the eye in patients with age-related macular degeneration]. Oftalmol Zh. 2013;3:23-26. Russian. 13.Jordana G. Fein, Lauren A. Branchini, Varsha Manjunath, Caio V. Regatieri, James G. Fujimoto, Jay S. Duker. Analysis of the Short Term Change in Subfoveal Choroidal Thickness in Eyes With Age Related Macular Degeneration Using Optical Coherence Tomography. Ophthalmic Surg Lasers Imaging Retina. 2014 Jan-Feb; 45(1): 32-37. 14.Age-related macular degeneration. NICE guideline [NG82]. Published date: January 2018. Available from: https://www.nice.org.uk/guidance/ng82 15.Vit VV. [The structure of the human visual system]. Odessa: Astroprint; 2018. 16.Arya M, Sabrosa AS, Duker JS, Waheed NK. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye Vis (Lond). 2018;5:22. 17.Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, Edwards MM, Lutty GA. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016 Nov 1;134(11):1272-1280. 18.Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562-2573. 19.Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45:2886-2892. 20.Korol AR, Zborovska O, Kustryn T, Dorokhova O, Pasyechnikova N. Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: A pilot study. Clinical Ophthalmology. 2017;11:1315-1320. 21.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996 Dec;37(13):2724-35.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript
|