J.ophthalmol.(Ukraine).2019;6:56-62.
|
http://doi.org/10.31288/oftalmolzh201965662 Received: 15 November 2019; Published on-line: 06 January 2020 Ultrastructural features of choroidal melanomas after 810-nm diode-laser transpupillary thermotherapy delivered using the developed methodology V.V. Vit, N.I. Molchaniuk, S.I. Poliakova, I.V. Tsukanova SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine"; Odesa (Ukraine) E-mail: inna.sister@gmail.com TO CITE THIS ARTICLE: Vit VV, Molchaniuk NI, Poliakova SI, Tsukanova IV. Ultrastructural features of choroidal melanomas after 810-nm diode-laser transpupillary thermotherapy delivered using the developed methodology. J.ophthalmol.(Ukraine).2019;6:56-62. http://doi.org/10.31288/oftalmolzh201965662
Background: An 810-nm diode-laser transpupillary thermotherapy (TTT) is used as monotherapy in the treatment of small choroidal melanomas (CM; measuring ? 3-4 mm in prominence). We developed an advanced methodology for performing the TTT, which involves four sessions delivered daily over four consecutive days. Purpose: To examine ultrastructural features of therapeutic pathomorphosis after TTT delivered using the developed methodology in enucleated eyes with choroidal melanoma in which eye-preserving treatment was not feasible. Material and Methods: Material for electron microscopy was obtained from enucleated eyes of seven CM patients who had been treated with TTT delivered over one to four sessions. One patient had undergone one, two had undergone two, two had undergone three, and two had undergone four daily TTT sessions before enucleation, with an 810-nm diode laser (Iridis Quantel medical, France) in a continuous mode, and the power was gradually increased from the initial 200 mW to about 1800 mW until the desired endpoint was achieved. Biopsy fragments were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4), post-fixed with 1% osmium tetroxide in phosphate buffer (pH 7.4), dehydrated through an ascending ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with lead citrate according to the procedure described by Reynolds, and observed with a PEM-100-01 Transmission Electron Microscope. Results: In our ultrastructural studies of choroidal melanoma after TTT delivered using the developed methodology, the most pronounced manifestations of therapeutic pathomorphosis were observed at day 4, and the developed methodology was substantiated by the ultrastructural findings. The therapeutic pathomorphosis in the melanoma tissue in response to 810-nm diode laser irradiation manifested itself as dry or wet necrosis, which was partial after the first treatment session, but a complete destruction of tumor cells at a depth of ? 4 mm from the irradiated surface was observed after four days of daily sessions. This indicates that our TTT delivery methodology can be used for treatment of small (T1) CM (measuring ? 3 mm in prominence and ? 12 mm in basal dimension). Conclusion: In our ultrastructural studies, the most pronounced changes in choroidal melanoma tissue manifested themselves as dry or wet necrosis, and were observed at day 4 of daily sessions of 810-nm diode laser TTT. Keywords: choroidal melanoma , diode-laser transpupillary thermotherapy, electron microscopy
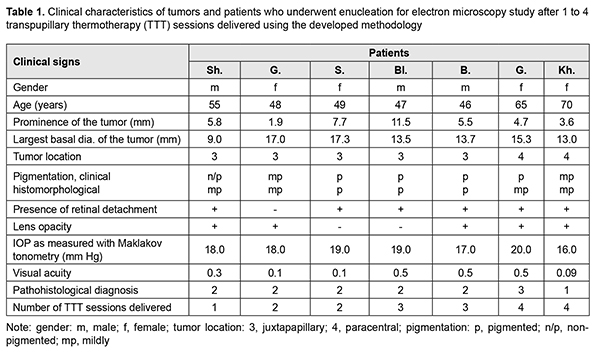
Introduction Nowadays, eye-preserving treatment is generally accepted as the first choice of treatment for patients with intraocular melanoma. An 810-nm diode laser transpupillary thermotherapy (TTT), either alone or in combination with brachytherapy, is used to treat for the small choroidal melanoma (CM; measuring ? 3 mm in prominence) [1-18]. We developed a methodology for performing the 810-nm diode laser TTT, which involves four sessions delivered daily over four consecutive days and, if required, one to four sessions 2.5 to 3 months thereafter [19]. The purpose of this study was to examine ultrastructural features of therapeutic pathomorphosis after TTT delivered using the developed methodology in enucleated eyes with choroidal melanoma in which eye-preserving treatment was not feasible. Material and Methods Material for electron microscopy was obtained from enucleated eyes of seven CM patients who had been treated with TTT delivered over one to four sessions. In these patients, enucleation of eyes with intraocular melanoma was performed for the following indications: the impossibility or patient refusal of eye-preserving treatment. All patients were explained the need for TTT before enucleation, and provided written informed consent for the study. The study was approved by the local bioethics committee (15 May 2003, Ref. No. 3). Biopsy fragments were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4), post-fixed with 1% osmium tetroxide in phosphate buffer (pH 7.4), dehydrated through an ascending ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with lead citrate according to the procedure described by Reynolds, and observed with a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine). One patient had undergone one, two had undergone two, two had undergone three, and two had undergone four daily TTT sessions before enucleation. TTT was performed on the eye with an 810-nm diode laser (Iridis Quantel medical, France) in a continuous mode, and the power was gradually increased from the initial 200 mW to about 1800 mW until the desired endpoint was achieved (either no color change or a subtle whitening of the tumor surfaces with no pain sensation). Laser spot size varied from 1.25 mm to 4 mm, the exposure time was 60 seconds, and the number of spots depended on the extent of the tumor. The clinical characteristics of patients and tumors are presented in Table 1.
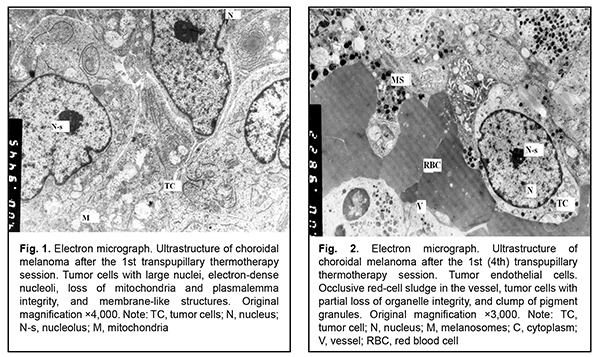
Results and Discussion Ultrastructural studies show that two types of cells (light cells and dark cells) differing in degree of destruction can be recognized in tumor tissue after TTT. Already after the first TTT treatment session, ultrastructural evaluation revealed loss of plasmalemma integrity, deep destruction of organelles, and partial or complete loss of nuclear chromatin in a portion of tumor tissue cells (i.e., there was ultrastructural evidence of total necrosis in these cells). In addition, certain cells showed focal necrosis with newly developed large electron-lucent cavities. Although these cells did have some organelles, they were also undergoing pathological changes (Fig. 1). In another portion of cells, nuclei and plasmalemma were better preserved, and there were numerous free ribosomes, which may be indicative of high protein synthesis in these cells. Membranous organelles (particularly, mitochondria), however, showed destruction, which was indicative of energy deficiency. Therefore, one could believe that these cells were also doomed to death (Figs. 2, 3). Of note is that some light cells possessed nuclei of practically normal ultrastructure, and some light tumor cells were located between endothelial cells of microvessels. Occlusive red-cell sludge was observed in the vessels.
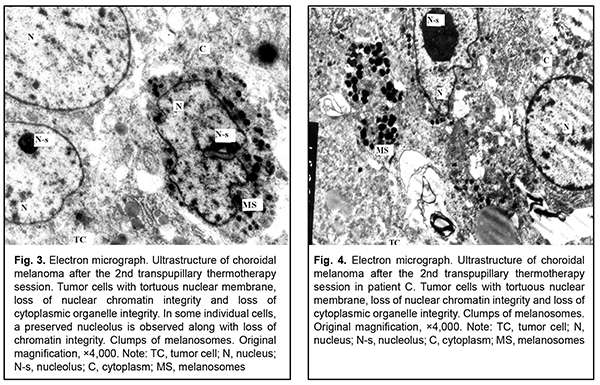
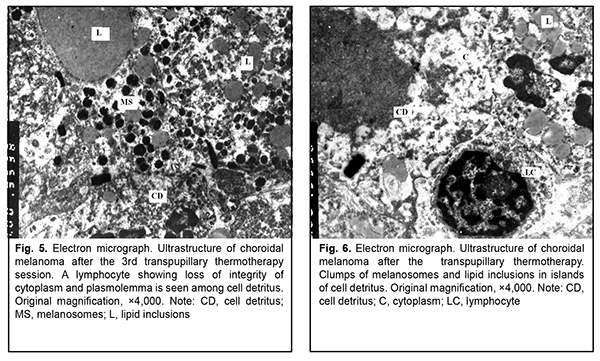
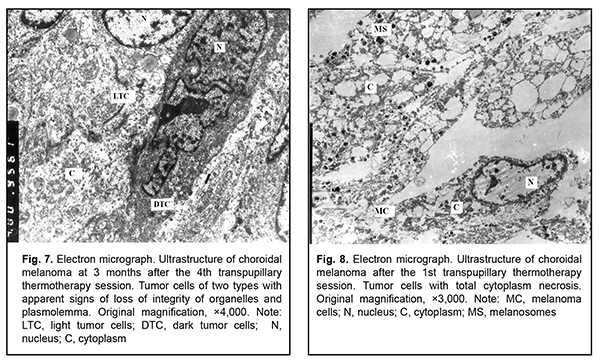
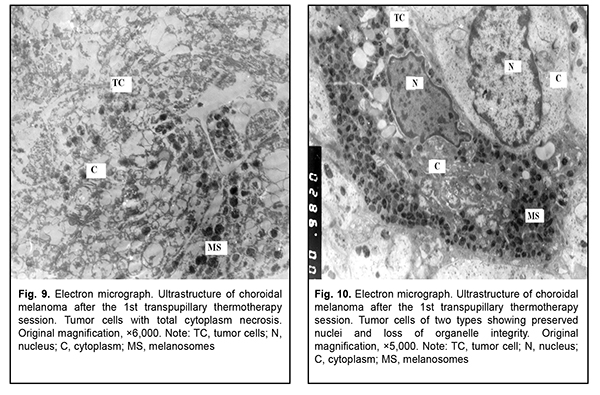
After the second TTT treatment session, the tumor tissue was represented by light cells with large round nuclei and a dark osmiophilic nucleolus. Disruption of plasmalemma and a relatively small cytoplasm surrounding the nucleus were observed in, and foci of large electron-lucent gaps between these cells (Fig. 4). Ultrastructurally, they had few membranous organelles in their cytoplasm, with some of these organelles showing destruction. Some cells showed tortuous nuclear membrane, pigment granules in the cytoplasm, and almost complete loss of nuclear chromatin (Fig. 5). Cells were mostly of a similar type. Cells with pigment granules were seen in between these cells. After the third TTT treatment session, the tumor tissue was represented mostly by loose cells with destruction of nucleus compartments and organelles, but not the nuclear membrane. Some cells contained pigment granules and lysosomes, but had no plasmalemma. Large electron-lucent gaps were observed between cells (Fig. 6). At some sites, cells with destruction of organelles appeared to be arranged in a layer and had no plasmalemma. Individual cells of another type had better preserved structure and were larger than the above cells. They exhibited nuclei with electron-dense karyoplasms and tortuous nuclear membrane, altered or completely destroyed cytoplasmic organelles and electron-dense cytoplasmic inclusions that could be pigment granules undergoing pathological changes. These cells were in contact with the light tumor cells whose cytoplasm was practically empty, but which contained preserved nuclei and nucleoli; the nucleoli showed pathological changes (Fig. 7). After the fourth TTT treatment session, the tumor tissue was represented mostly by loose and completely destroyed cells. In between these cells, there were lipid droplets of various shape and size and myelin-like coils formed as a result of disruption of cell membrane structures. Some ghost cells showed preserved nuclei; however, since their cytoplasmic structures were broken down, these cells were doomed to break up (Fig. 8). Small islands of cell detritus and aggregates of round pigment granules and lipid inclusions of various sizes were occasionally seen (Fig. 9). Lymphocytes showing loss of integrity of cytoplasm and plasmolemma were revealed among disintegrated cells (Fig. 10). Therefore, in our ultrastructural studies of choroidal melanoma after TTT delivered using the developed methodology, the most pronounced manifestations of therapeutic pathomorphosis were observed at day 4, and the developed methodology was substantiated by the ultrastructural findings. The therapeutic pathomorphosis in the melanoma tissue in response to 810-nm diode laser irradiation manifested itself as dry or wet necrosis, which was partial after the first treatment session, but a complete destruction of tumor cells at a depth of ? 4 mm from the irradiated surface was observed after four days of daily sessions. This indicates that our TTT delivery methodology can be used for treatment of small (T1) CM (measuring ? 3 mm in prominence and ? 12 mm in basal dimension). It has been reported that ultrastructural post-TTT changes manifested themselves as dry or wet necrosis of the tumor tissue [20-25], with a depth of necrosis of 2.2 mm [1] to 4.7 mm [26]. Some investigators believe that TTT is of limited value as a monotherapy against mildly pigmented or non-pigmented choroidal melanoma. Although we cannot reject their opinion, in the current study, there was ultrastructural evidence of tumor tissue destruction in mildly pigmented and non-pigmented tumors as early as the first days of daily TTT sessions. In addition, failures in TTT treatment of choroidal melanoma can be partially explained by the cell type of cancer origin. Conclusion In our ultrastructural studies, the most pronounced changes in choroidal melanoma tissue manifested themselves as dry or wet necrosis, and were observed at day 4 of daily sessions of 810-nm diode laser TTT. References 1.Bulgakova ES. [Treatment of small choroidal melanomas with diode laser transpupillary thermotherapy]. [Abstract of Cand Sc (Med) Thesis]. Moscow: Helmholtz Research Institute for Eye Diseases; 2005. Russian. 2.Linnik LF, Magaramov DA, Iarovoi AA, et al. [Potential of diode laser transpupillary thermotherapy as an organ- and function-preserving treatment option in small choroidal melanomas]. In: [Proceedings of the Jubilee Symposium on Current Issues in Ophthalmology]. Research Institute of Eye Disease: Moscow; 2003. p.86-7. Russian. 3.Linnik LF, Magaramov DA, Iarovoi AA, Semikova TS. [Diode laser transpupillary thermotherapy: the potential for the treatment of small choroidal melanomas]. Ophthalmokhirurgiia. 2002;(3):45–50. Russian. 4.Iarovoi AA, Linnik LF, Magaramov DA, et al. [Diode laser transpupillary thermotherapy: the potential for the treatment of small choroidal melanomas]. Russkii meditsinskii zhurnal. 2004;5(2):77–81. Russian. 5.Linnik LF, Magaramov DA, Iarovoi AA, et al. [Three year experience of using diode laser transpupillary thermotherapy as a monotherapy for the management of uveal melanoma]. Ophthalmokhirurgiia. 2003;(4):17–24. Russian. 6.Iarovoi AA, Linnik LF, Semikova TS, Bulgakova ES. [Small choroidal melanomas: clinical picture and selecting the appropriate treatment option]. Novoiie v ophthalmologii. 2004;(2):28–37. Russian. 7.Aaberg TM Jr, Bergstrom CS, Hickner ZJ. Long term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol. 2008 Jun;92(6):741-6. 8.De Potter P, Levecq L. [Transpupillary thermotherapy in the treatment of choroid melanoma]. J Fr Ophtalmology. 2001 Nov;24(9):937-43. French. 9.De Potter P. [Choroidal melanoma: current therapeutic approaches]. J Fr Ophtalmology. 2002 Feb;25(2):203-11. French. 10.Duquesne N, Hajji Z, Jean-Louis B, Grange JD. [Choroidal nevi associated with serous macular detachment]. J Fr Ophthalmol. 2002 Apr;25(4):393-8. French. 11.Forte R, Cennamo G. [Transpupillary thermotherapy of choroidal melanomas]. J Fr Ophtalmol. 2008 Mar;31(3):279-81. French. 12.Godfrey DG, Waldron RG, Capone A Jr. Transpupillary thermotherapy for small choroidal melanoma. Am J Ophthalmol. 1999 Jul;128(1):88-93. 13.Pan Y, Diddie K, Lim JI. Primary Transpupillary Thermotherapy for Small Choroidal Melanomas. Br J Ophthalmol. 2008 Jun;92(6):747-50. 14.Chojniak MM, Chojniak R, Nishimoto IN, Allemann N, Erwenne CM. Primary transpupillary thermotherapy for small choroidal melanoma. Graefes Arch Clin Exp Ophthalmol. 2011 Dec;249(12):1859-65. 15.Shields CL, Shields JA, Peres N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002 Feb;109(2):225-34. 16.Primavera V, Russo V, Laculli C, Delle Noci N. Transpupillary thermotherapy for small choroidal melanoma: results in 25 patients. Xth International congress of ocular oncology: Final program and abstract book. Amsterdam, the Netherlands; 2001. p. 292. 17.Robertson DM, Buettner H, Bennett SR. Transpupillary thermotherapy as primary treatment for small choroidal melanomas. Arch Ophthalmol. 1999 Nov;117(11):1512-9. 18.Turcotte S, Bergeron D, Rousseau AP, Mouriaux F. Primary transpupillary thermotherapy for choroidal indeterminate melanocytic lesions. Can J Ophthalmol. 2014 Oct;49(5):464-7. 19.Information Bulletin No. 22, p.3, based on Pat. of Ukraine №102,890 issued 25.11.2015. Method for treatment of T1 choroidal melanoma. Authors: Pasyechnikova NV, Naumenko VO, Poliakova SI, Tsukanova IV. Owner: State Institution Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine. Ukrainian. 20.Diaz CE, Capone A, Grossniklaus HE. Clinicopathologic findings in recurrent choroidal melanoma after transpupillary thermotherapy. Ophthalmology. 1998 Aug;105(8):1419-24. 21.Наrbour JW, Meredith TA, Thompson PA, Gordon ME. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003 Nov;110(11):2207-15. 22.Journee-de Korver JG, Oosterhuis JA, Kakebeeke-Kemme HM, Wolff-Rouendaal D. Transpupillary thermotherapy (TTT) by infrared irradiation of choroidal melanoma. Doc Ophthalmol. 1992;82(3):185-91. 23.Singh AD, Eagle RC Jr, Shields CL, Shields JA. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003 Mar;121(3):397-400. 24.Shields CL, Shields JA, DePotter P, Kheterpal S. Transpupillary thermotherapy in the management of choroidal melanoma. Ophthalmology. 1996 Oct;103(10):1642-50. 25.Shields CL, Shields JA, Cater J, et al. Transpupillary thermotherapy choroidal melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998 Apr;105(4):581-90. 26.Langmann G, Kleinert R, Faulbom J, et al. [Transpupillary diode laser hyperthermia histopathology findings of eyes with melanoma and first clinical results]. Abstract. Retinologische Gesellschaft, Munchen, Germany. June, 1996.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|