J.ophthalmol.(Ukraine).2019;5:49-55.
|
Received: 24 June 2019; Published on-line: 30 October 2019 Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 3. Vitreous cavity irrigation with 36°С solution O.S. Zadorozhnyy, Cand Sc (Med), R.E. Nazaretian, V.A. Naumenko, Dr Sc (Med), Prof., E.V. Maltsev, Dr Sc (Med), Prof., N.V. Pasyechnikova, Dr Sc (Med), Prof, Corr Member of NAMS of Ukraine Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) E-mail: laserfilatova@gmail.com TO CITE THIS ARTICLE: Zadorozhnyy OS, Nazaretian RE, Naumenko VA, Maltsev EV, Pasyechnikova NV. Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 3. Vitreous cavity irrigation with 36°С solution. J.ophthalmol.(Ukraine).2019;5:49-55. http://doi.org/10.31288/oftalmolzh201954955
Background: Currently, there are no clear recommendations on temperature modes regarding safety for ocular fundus structures during vitrectomy. Our rabbit study has previously found that formation of numerous vacuoles in retinal layers represents the most apparent retinal changes after vitrectomy with prolonged irrigation with low-temperature (22°С or 5°С) fluids. Purpose: To investigate the structure of the rabbit retina and choroid after vitrectomy with prolonged irrigation with 36°С fluid. Materials and Methods: Twelve rabbits (24 eyes) were included in this study and divided into the experimental group (10 animals, 20 eyes) and the control group (2 intact animals, 4 eyes). The former group had vitrectomy with 30-minute or 60-minute irrigation with 36°С Balanced Ringer’s lactate. Vitrectomy was performed using the Alcon Accurus 400VS vitrectomy system (Alcon Laboratories). Histological studies of the choreoretinal complex (the retina and choroid) were performed at days 1 and 7 after surgery. Results: At baseline, mean rectal temperature was 38.3 ± 0.3 °С. Mean midvitreous temperature decreased from 37.4 ± 0.2°С at baseline to 36.4±0.1°С (i.e., by 1 °С) (p < 0.0002). Histological examination found no apparent vacuolization of retinal structures at the microscopic slides obtained after prolonged surgery performed under conditions of mild hypothermia compared to eyes operated under conditions of deep hypothermia with 22°С and especially 5°С fluids. Conclusion: In rabbits, the use of 36°С irrigation fluids during vitrectomy resulted in a decrease in vitreal cavity temperature to the level of mild hypothermia, with no structural changes in the chorioretinal complex within 7 postoperative days after prolonged irrigation of the vitreous cavity. A reasonable assumption can be made that, after vitrectomy with prolonged irrigation of the vitreous cavity (with the 22°С or 5°С solutions) under conditions of deep hypothermia, apparent vacuolization developed mostly due to the low-temperature effect of irrigating fluid. There is need for further research aimed at identification of the safest modes for use of local ocular hypothermia during vitrectomy and investigation of optimum temperature conditions in the postoperative period. Keywords:vitrectomy, intraocular temperature, rabbit eye, choreoretinal complex
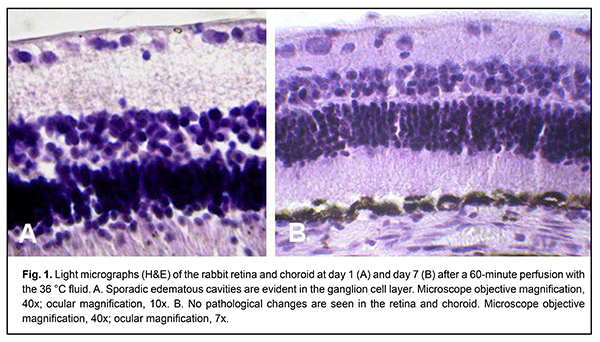
Introduction Pars plana vitrectomy is widely used in the treatment of rhegmatogenous retinal detachments, proliferative diabetic retinopathy and other vitreoretinal disorders. The surgical intervention (e.g., in severe proliferative diabetic retinopathy) may be time consuming for the performing surgeon, often taking 60 minutes or more [1]. Despite continuous advances in vitrectomy technology, there still remains a wide range of challenges affecting treatment outcomes [2-7]. The temperature of irrigation solutions used during vitrectomy is substantially lower than that of the intraocular media, and neither of these temperatures is commonly monitored during the procedure [8]. Consequently, vitrectomy is performed under conditions of uncontrolled local hypothermia. Moreover, there are no clear recommendations on temperature modes regarding safety for ocular fundus structures during vitrectomy. Therapeutic controlled hypothermia has been successfully applied in other medical fields (like cardiac surgery, neurosurgery, and resuscitation science) for improving brain cell resistance to ischemic conditions, and there are relevant recommendations for these fields [9, 10]. Hypothermia can be classified to mild (32-35°C), moderate (28-32°C) and deep (< 28°C) [11], based on the depth of cooling from a normal body temperature of 37-38°C, and may result in either positive or negative consequences depending on the amount of temperature decrease, duration of the low-temperature effect, and rate of tissue rewarming [12-15]. Although there is no uniform protocol to administer induced hypothermia in intraocular surgery, some authors believe it reasonable to use low-temperature irrigating fluids during vitrectomy [16-18]. Our rabbit study [19] has previously found that formation of numerous vacuoles in retinal layers (especially the internal retinal layers) represents the most apparent retinal changes after vitrectomy with prolonged irrigation with low-temperature (22°С or 5°С) fluids. The question arises of whether this phenomenon is caused by a decrease in irrigating fluid temperature or other factors such as mechanical impact or irrigating fluid composition. The purpose of the study was to investigate the structure of the rabbit retina and choroid after vitrectomy with durable irrigation with 36°С fluid. Materials and Methods Twelve Chinchilla rabbits (24 eyes; weight, 2.5-3.5 kg) were included in this study and divided into the experimental group (10 rabbits, 20 eyes) and the control group (2 intact rabbits, 4 eyes). Experimental animals had vitrectomy with 36°С irrigating fluid. The ambient operating room temperature was between 22 °С and 24 °С. A 23-G three-port pars plana vitrectomy was performed using the Alcon Accurus 400VS vitrectomy system (Alcon Laboratories, Fort Worth, TX). Technique The surgical site was prepared with antiseptic solution and epibulbar anesthetic was administered. Thereafter, core and peripheral vitrectomy was performed with cutting rates of 1500-1800 cuts/min, aspiration pressure of 150 mm Hg, and irrigation pressure of 20 mm Hg. Mean vitrectomy time was 4 minutes, and mean irrigation/aspiration time was 30 minutes or 60 minutes. Balanced Ringer’s lactate was used as an intraocular irrigating solution. Warm (36 °С) fluid was prepared by warming the solution inside the irrigating tube with gel packs that were located outside the tube, and thus warming was performed in close proximity to the surgical site. The temperature of the irrigating solution delivered into the eye was monitored and controlled during surgery. A thermoelectric device developed by the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring temperatures of various ocular structures and irrigating solution [20]. After a lid speculum was placed, epibulbar anesthetic was administered, the three ports were created, and a temperature measuring probe was introduced into the vitreous cavity through a standard 23-G pars plana port. Midvitreous temperatures were measured before surgery and at different surgery time points. In addition, rectal temperature measurements were taken, and air temperature in the operating room was registered. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. The animals were housed and bred conventionally. Each animal underwent biomicroscopy and ophthalmoscopy at baseline, immediately after surgery, and then daily until euthanasia. Prior to surgery, animals were anesthetized with thiopental sodium 10% (1.0 mL/kg, intramuscularly). Immediately thereafter, both eyes received a drop of proxymetacaine HCl (0.5%) for topical anesthesia. The pupils were dilated with atropine sulphate. After surgery, a drop of sulfacyl natrium 20% and a drop of Ofloxacin 0.3% were applied to each eye four times daily during the observation period of 1 to 7 days. Histology was performed at the Pathology and Electronic Microscopy Laboratory of the Filatov Institute. Animals were euthanized at days 1 and 7 after surgery. Enucleated globes were fixed and embedded in paraf?n, and serial sections (5 ?m thick) were cut, deparaf?nized, and stained with hematoxylin and eosin in a routine manner. The chorioretinal complex was histologically examined by light microscopy. Statistical analysis The experimental temperature data was subjected to statistical analysis. Data is presented as mean ± standard deviation (SD). Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ? 0.05 was assumed. Results At day 1 after vitrectomy with 30-minute vitreous irrigation with 36 °С fluid, histological examination found no loss of the normal layered structure of the retina. Specifically, no changes were found in the number of cell rows in the inner nuclear layer (INL) and outer nuclear layer (ONL), and (2) structure of the inner and outer plexiform layers (the width of the former being greater than that of the latter). The visual streak appeared normal, containing increased number of ganglion neurons, and the medullary rays were vascularized. No pathological changes were found in the retinal pigment epithelium layer. Sporadic edematous cavities were observed at some locations on the slides, e.g., in the ganglion cell layer. No structural changes or edema phenomena were found in the choroid. At day 7, retinal histology remained normal. At day 1 after vitrectomy with 60-minute vitreous irrigation with 36 °С fluid, all retinal layers and structures were clearly visualized by light microscopy (Fig. 1A). In addition, RPE cells contained much pigment, and the layer width ratio was normal for all the layers. Moreover, the visual streak was normal, containing a high number of ganglion neurons; the medullary rays were vascularized; and no changes were found in the number of cell rows in the ONL and INL layer (up to 8-10 rows and 4-5 rows, respectively). Although no apparent edema was noticed, sporadic edematous cavities were observed at some locations (e.g., in the ganglion cell layer and photoreceptor layer) on the slides, and the choroid appeared somewhat thickened. At day 7, retinal histology remained normal, and the retinal structures appeared identical to those found in intact animals (Fig. 1B).
At baseline, mean rectal temperature was 38.3 ± 0.3 °С. Mean midvitreous temperature decreased from 37.4 ± 0.2°С at baseline to 36.4±0.1°С during vitreous cavity irrigation, but was not significantly different from baseline as early as 10 minutes after completion of irrigation. No corneal changes were observed, and lens clarity was maintained in all eyes during surgery. Discussion It is now recognized that a change in body temperature as small as 1°C can cause a number of useful or harmful reactions [21]. A beneficial effect of mild hypothermia on brain neurons has been demonstrated in models of ischemia. Mild hyperthermia, however, potentiates damage to the brain, producing effects similar to those observed in increased duration of ischemia [22, 23]. In addition, mild hypothermia prevents blood–brain barrier disruption and decreases the risk of the development of brain edema under cerebral ischemic conditions [24], and inhibits pro-inflammatory brain tissue responses [25]. Moreover, moderate low temperature has been reported to suppress apoptosis of the cells [26]. In patients who have been successfully resuscitated after cardiac arrest due to ventricular fibrillation, therapeutic mild hypothermia increased the rate of a favorable neurologic outcome and reduced mortality [10, 12]. Hyperthermia after cardiac arrest is, however, associated with unfavorable neurologic outcome [13, 27]. Therefore, mild hypothermia has a beneficial effect on neural tissue structure and function under ischemic conditions, whereas mild hyperthermia demonstrates the opposite effect. In rabbits and humans, the temperature of the vitreous cavity is always somewhat lower than that of the body [8, 20, 28, 29]. Vitreous temperatures undergo changes in the course of vitrectomy depending on irrigating fluid temperature. Consequently, with the use of irrigating fluid temperature equal to that of the body, surgery is performed under conditions of mild hyperthermia, which may have an unfavorable effect on the structure of retinal tissue, especially in the presence of ischemia. Vitrectomy is usually performed in patients who already have ischemic retinal injury, e.g., patients with diabetic retinopathy. In addition, IOP increase and systemic arterial pressure decrease during vitrectomy can result in decreased perfusion pressure, leading to additional intraoperative ischemic retinal and optic nerve injury [5]. Tamai and colleagues [17] confirmed the destructive effects of mild hyperthermia, and demonstrated neuroprotective effects of hypothermia on retinal structures under ischemic conditions. In that study, after the rabbits underwent closed vitrectomy, their vitreous cavities were continuously irrigated with 8°С, 22°С or 38°С solution for 30 minutes at a perfusion pressure of 140 mm Hg to induce ischemia in the rabbit eye. At day 7, retinal damage in the 38°C group revealed more severe histological impairment than in either of the two other treatment groups or controls. Therefore, under ischemic conditions, even mild hyperthermia is dangerous not only for brain nerve cells, but also for the retina. As hyperthermia poses danger during vitrectomy, it is consequently reasonable to use normothermic or hypothermic irrigation fluids for prolonged surgery, especially since the beneficial features of hypothermia are quite well known. In addition, questions rise regarding safe levels and duration of exposure to hypothermia in the course of surgery. The damaging effects of deep hypothermia on nerve tissue structure have been reported in experimental animals. Dal? and colleagues [30] observed severe neuronal cell damage characterized by swelling, vacuolated cytoplasm with distended neuronal bodies and eccentric nuclear displacement in amphibians in the presence of deep hypothermia. They supposed that excitotoxicity induced by deep hypothermia conditions underlies this damage. We have previously demonstrated in rabbits that the use of room-temperature and cooler (e.g., 22°С and 5°С, respectively) solutions in vitreoretinal surgery resulted in vitreous temperature drops of more than 11°С and 26°С, compared to baseline levels. Therefore, deep hypothermia was observed during vitrectomy in rabbits even in the use of room-temperature (22°С) irrigation solutions. In addition, light microscopy revealed that continuous 60-minute irrigation of the vitreous cavity with the 22°С and especially 5 °C solution during vitrectomy resulted in retinal structural changes in the form of uneven edema in the inner and outer retinal layers [19]. Moreover, continuous 30-minute irrigation of the vitreous cavity with the solutions of the same temperatures during vitrectomy resulted only in isolated foci of vacuolization in the ganglion cell layer [31]. Others have also reported on retinal damage in experimental animals after vitrectomy with prolonged vitreous cavity irrigation with low-temperature (2°С) fluids [32]. A study by Horiguchi and co-authors [33] has shown that the use of room-temperature solutions in surgery resulted in marked reduction in electrical activity of the human retina. Abnormal electroretinograph readings normalize with an increase in the temperature of irrigating solution to 34°С, i.e., to the level of normothermia or mild hypothermia, because at baseline, the human vitreous temperature at that study was 34-35°С. Therefore, a decrease in vitreous cavity temperature to the level of deep hypothermia during vitrectomy (and especially prolonged vitrectomy) may be unsafe. Nevertheless, in heart surgery, moderate hypothermia has been widely used for brain protection, and deep hypothermia has been used to correct severe aortic arch heart disease [14]. Although it is likely that some cases would benefit from the use of relatively deep cooling in vitrectomy, this warrants further research. In the current study, mean core body temperature and mean vitreous temperature were 38.3±0.3°С and 37.4±0.2°С, respectively, at baseline, and mean vitreous temperature decreased to 36.4±0.1°С, i.e. by 1.0°С (p < 0.0002), with the use of 36°С irrigating fluid during vitrectomy. Therefore, these surgical interventions were performed under conditions of mild hypothermia. Histological examination found no apparent vacuolization of retinal structures at the microscopic slides after prolonged vitrectomy performed under conditions of mild hypothermia compared to eyes operated under conditions of deep hypothermia [19]. A reasonable assumption can be made that numerous vacuoles appeared after prolonged vitrectomy mostly due to the effect of low temperature, and to a less extent to those of mechanical damage or irrigating fluid composition. Since mild hypothermia exerts several positive effects on nerve tissue structures and does not result in cold-related damage to retinal tissue, we find it reasonable to recommend it for prolonged surgery. This is in agreement with others [34] who proposed to use warmed irrigation fluid for achieving mild hypothermia during surgery. The retinal changes observed following vitrectomy might be explained not only by cooling of the intraocular structures during vitrectomy, but also by their quick rewarming after surgery. The process of tissue rewarming after cooling is given special importance in critical care practice, since quick warming up contributes to nervous system injury [12, 27]. We have demonstrated previously that intraocular temperatures return to baseline levels following vitrectomy much faster than recommended for critical conditions by intensive therapy guidelines, which may result in additional damage to retinal cells [15, 27, 29]. Temperature monitoring is required also after the cooling stage. It is known from the practice of critical care intensive therapy that patients who developed even mild hyperthermia exhibited much more significant derangement of cerebrovascular reactivity, indicating the need for maintaining controlled normothermia after the cooling stage [35]. Corneal temperature measures after glaucoma surgery [36] and after cataract surgery [37] were higher compared to baseline values. Therefore, local ocular hypothermia developing postoperatively might be a factor of retinal damage. Conclusion First, in rabbits, the use of 36°С irrigation fluids during vitrectomy resulted in a decrease in vitreal cavity temperature to the level of mild hypothermia, with a decrease in vitreous sample temperature from 37.4±0.2°С at baseline to 36.4±0.1°С. Second, continuous 30-minute or 60-minute irrigation of the vitreous cavity with the 36°С solution during vitrectomy did not result in structural changes in the chorioretinal complex within 7 postoperative days. A reasonable assumption can be made that, after vitrectomy with prolonged irrigation of the vitreous cavity (with the 22°С or 5°С solutions) under conditions of deep hypothermia, apparent vacuolization developed mostly due to the low-temperature effect of irrigating fluid. Finally, there is need for further research aimed at identification of the safest modes for use of local ocular hypothermia during vitrectomy and investigation of optimum temperature conditions in the postoperative period. References 1.Shishkin MM, Yuldasheva NM. [Intravitreal injection of angiogenesis inhibitors as a stage of sparing vitreoretinal surgery of proliferative diabetic retinopathy]. Vestnik Natsionalnogo medico-khirurgicheskogo tsentra im. Pitogova. 6(1): 77-81. Russian. 2.Postel EA, Pulido JS, Byrnes GA, Heier J, Waterhouse W, Han DP, et al. Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol. 1998 Jun;116(6):753-7. 3.Farah M, Maia M, Rodrigues EB. Dyes in Ocular Surgery: Principles for Use in Chromovitrectomy. Am J Ophthalmol. 2009 Sep;148(3):332-40. 4.Hasumura T, Yonemura N, Hirata A, Murata Y, Negi A. Retinal Damage by Air Infusion during Vitrectomy in Rabbit Eyes. Invest Ophthalmol Vis Sci. 2000 Dec;41(13):4300-4. 5.Rossi T, Querzoli G, Angelini G, Rossi A, Malvasi C, Iossa M, et al. Ocular perfusion pressure during pars plana vitrectomy: a pilot study. Invest Ophthalmol Vis Sci. 2014 Dec 2;55(12):8497-50. 6.Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH. Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg. 2001 Mar;27(3):437-44. 7.Goto A, Inatani M, Inoue T, Awai-Kasaoka N, Takihara Y, Ito Y, et al. Frequency and risk factors for neovascular glaucoma after vitrectomy in eyes with proliferative diabetic retinopathy. J Glaucoma. 2013 Sep;22(7):572-6. 8.Iguchi Y, Asami T, Ueno S, Ushida H, Maruko R, Oiwa K, et al. Changes in vitreous temperature during intravitreal surgery. Invest Ophthalmol Vis Sci. 2014 Apr 11;55(4):2344-9. 9.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006 Sep;70(3):369-80. 10.The Hypothermia after Cardiac Arrest Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 Feb 21;346(8):549-56. 11.Saad H, Aladawy M. Temperature management in cardiac surgery. Glob Cardiol Sci Pract. 2013; 2013(1): 44–62. 12.Nunnally ME, Jaeschke R, Bellingan GJ, Lacroix J, Mourvillier B, Rodriguez-Vega GM, et al. Targeted temperature management in critical care: A report and recommendations from five professional societies. Crit Care Med. 2011 May;39(5):1113-25. 13.Lundbye JB. Therapeutic hypothermia after cardiac arrest. Clinical application and management. London: Springer; 2012. 14.Lomivorotov VN. [Hypothermic protection of the brain in cardiac surgery]. Patologiia krovoobrashcheniia i kardiokhirurgiia. 2010;3:7-10. Russian. 15.Tsarev A V. [Target temperature management in the clinical practice of critical care intensive therapy]. Meditsina neotlozhnykh sostoianii. 2014;7:186-91. Russian. 16.Rinkoff J, Machemer R, Hida T, Chandler D. Temperature-dependent light damage to the retina. Am J Ophthalmol. 1986 Oct 15;102(4):452-62. 17.Tamai K, Toumoto E, Majima A. Local hypothermia protects the retina from ischaemic injury in vitrectomy. Brit J Ophthalmol. 1997 Sep; 81(9): 789–94. 18.Jabbour NM, Schepens CL, Buzney SM. Local ocular hypothermia in experimental intraocular surgery. Ophthalmology. 1988 Dec;95(12):1687-90. 19.Zadorozhnyy O, Nazaretian R, Myrnenko V, Naumenko V, Maltsev E., Pasyechnikova N. Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 2. Vitreous cavity irrigation with different temperature solutions for 60 minutes. J Ophthalmol (Ukraine). 2018;4:49-55. 20.Anatychuk L, Pasyechnikova N, Zadorozhnyy O, Nazaretian R, Myrnenko V, Kobylyanskyi R, Gavrilyuk N. Original device and approaches to the study of temperature distribution in various eye segments (experimental study). J Ophthalmol (Ukraine). 2015;6:50-53. 21.Kochanek PM, Jackson TC. The Brain and Hypothermia-From Aristotle to Targeted Temperature Management. Crit Care Med. 2017 Feb;45(2):305-310. 22.Minamisawa H, Smith M, Siesj? BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990 Jul;28(1):26-33. 23.White MG, Luca LE, Nonner D, Saleh O, Hu B, Barrett EF, et al. Cellular mechanisms of neuronal damage from hyperthermia. Prog Brain Res. 2007;162:347-71. 24.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012 Feb 22;13(4):267-78. 25.Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003 Oct;34(10):2495-501. 26.Saito K, Fukuda N, Matsumoto T, Iribe Y, Tsunemi A, Kazama T, Yoshida-Noro C, Hayashi N. Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein. Brain Res. 2010 Oct 28;1358:20-9. 27.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods. Crit Care Med. 2009 Mar;37(3):1101-20. 28.Schwartz B, Feller MR. Temperature gradients in the rabbit eye. Invest Ophthalmol. 1962 Aug;1:513-21. 29.Anatychuk LI, Pasyechnikova NV, Naumenko VA, Nazaretian RE, Umanets M.M., Kobylianskyi RR, Zadorozhnyy OS. Intraoperative changes in intraocular temperature during vitrectomy procedures with irrigating solutions differing in temperature. J Ophthalmol (Ukraine). 2019;1:33-8. 30.Dal? NL, Bracho GA, Pi?a-Crespo JC. Motor impairment and neuronal damage following hypothermia in tropical amphibians. Int J Exp Pathol. 2007 Feb;88(1):1-7. 31.Zadorozhnyy O, Nazaretian R, Myrnenko V, Naumenko V, Maltsev E, Pasyechnikova N. Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 1. Vitreous cavity irrigation with different temperature solutions for 30 minutes. J Ophthalmol (Ukraine). 2018;3:80-4. 32.Zilis J.D., Chandler D., Machemer R. Clinical and Histologic Effects of Extreme Intraocular Hypothermia. Am J Ophthalmol. 1990 Apr 15;109(4):469-73. 33.Horiguchi M., Miyake Y. Effect of Temperature on Electroretinograph Readings During Closed Vitrectomy in Humans. Arch Ophthalmol. 1991 Aug;109(8):1127-9. 34.Mauro A, Massarotti N, Salahudeen M, Cuomo F, Costagliola C, Ambrosone L, Romanо MR.. Design of a novel heating device for infusion fluids in vitrectomy. Appl Therm Eng. 2018; 128:625-36. 35.Lavinio A, Timofeev I, Nortje J, Outtrim J, Smielewski P, Gupta A, et al. Cerebrovascular reactivity during hypothermia and rewarming. Br J Anaesth. 2007 Aug;99(2):237-44. 36.Galassi F, Giambene B, Corvi A, Falaschi G, Menchini U. Retrobulbar hemodynamics and corneal surface temperature in glaucoma surgery. Int Ophthalmol. 2008 Dec;28(6):399-405. 37.Fujishima H, Toda I, Yagi Y, Tsubota K. Quantitative evaluation of postsurgical inflammation by infrared radiation thermometer and laser flare-cell meter. J Cataract Refr Surg. 1994 Jul;20(4):451-4.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|