J.ophthalmol.(Ukraine).2019;5:37-41.
|
http://doi.org/10.31288/oftalmolzh201953741 Received: 16 July 2019; Published on-line: 30 October 2019 Status of humoral immune factors in peripheral blood of fronto-orbital trauma patients late after surgery with the use of biocomposite О.D. Bondarchuk1,2, V.V. Kishchuk1, О.F. Melnykov3, М.D. Tymchenko3, N.D. Didyk2 1 Pirogov National Medical University of Vinnytsia; Vinnytsia (Ukraine) 2 Pirogov Regional Clinical Hospital of Vinnytsia; Vinnytsia (Ukraine) 3 Prof. Kolomiichenko Institute of Otolaryngology, NAMS of Ukraine; Kyiv (Ukraine) E-mail: eskulap20009@gmail.com TO CITE THIS ARTICLE: Bondarchuk О.D., Kishchuk V.V., Melnykov О.F., Tymchenko М.D., Didyk N.D. Status of humoral immune factors in peripheral blood of fronto-orbital trauma patients late after surgery with the use of biocomposite. J.ophthalmol.(Ukraine).2019;5:37-41. http://doi.org/10.31288/oftalmolzh201953741
Purpose: To identify the influence of biocomposite on immune system status in front bone trauma (FBT) patients at 9 months or later after surgery involving the use of the composite material. Material and Methods: Twenty FBT patients underwent an examination 9 months or later after surgery. They were divided into two groups, group A with a problematic clinical course and group B with an uneventful clinical course during follow-up. The control group was composed of 10 age-matched and practically healthy individuals. Serum levels of immunoglobulin (Ig)M, IgG, IgA and IgE, interleukins (IL)-1 and IL-10, transforming growth factor (TGF)-?, circulating immune complexes (CIC), and gamma interferon (IFN-?), and agglutination antibodies to antigens of allogeneic connective tissue were measured. Non-parametric Wilcoxon U test and Student t-test were used for statistical analysis. Results: Activation of pro- and anti-inflammatory interleukins and no changes in the levels of immunoglobulins and CIC were found in group A, but not group B or the controls. In addition, patients from group A exhibited decreased serum levels of TGF-? and IFN-?, which may be considered as pathogenetically significant factors involved in regeneration processes. No humoral autoimmune response to connective tissue antigens was found. Conclusion: Two disease course variants were found in the late phase after FBT. Increased serum levels of pro- and anti-inflammatory interleukins and decreased serum levels of TGF-? and, especially, IFN-?, were observed in patients with problematic regeneration. No humoral autoimmune response to connective tissue antigens was found in either group of patients with FBT. Stress mechanisms might be involved in decreased immunity levels in these patients. Keywords: trauma, immunoglobulins, cytokines, growth factor, immune complexes, humoral autoimmune responses
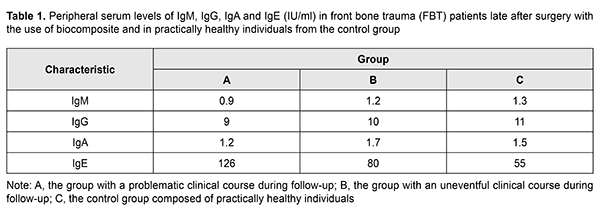
Introduction A substantial immune system response to skull trauma is observed both early and late after skull trauma, with changes in immune reaction levels in the early post-injury phase, and possible development of autoimmune reactions in the late post-injury phase [1-4]. Hydroxyapatite-based biocomposites (such as SYNTEKIST) have been used in surgical treatments for front bone trauma (FBT) in recent years, and are capable of facilitating ostheogenesis and preventing liquorrhea and other post-traumatic sequelae [2, 5, 6]. It is reasonable and necessary to assess the possibility for the development of autoimmune processes to connective tissue antigens, chemical components of SYNTEKIST material, lymphoid tissue antigens, and humoral immunity status [7-10]. Materials of a SYNTEKIST biocomposite group have the following feature: they are bioactive multiphase inorganic composite materials which contain a superposition of virtually all phase and chemical components available in domestic and foreign materials of similar purpose which, in contrast, contain only one or two components. In addition, they contain some new phase and chemical components and their combinations [11-13]. This makes it possible to plan rather accurately the interaction of these materials with body tissues and to predict, for instance, the formation of pores in the material after implantation or a type of implant biodegradation, to adjust the strength, osteoconductive properties, and the rate of absorption of the material or its individual components, and to improve mechanic properties of implants and regulate the change in these properties with time [5, 6]. The purpose of the study was to measure humoral immune factors in the peripheral blood of patients with fronto-orbital trauma, particularly, frontal bone trauma, at late time points (9 months or later) after surgery with the use of SYNTEKIST biocomposite. Materials and Methods Twenty patients (14 men and 6 women; age, 14 to 65 years) with frontal bone trauma (FBT) underwent an examination 9 months or later after surgery involving the use of SYNTEKIST biocomposite. They were divided into two groups, group A (10 patients) with a problematic clinical course (untimely healing of wound, with a soft tissue response in the form of sponginess and mild hyperemia, and, in some cases, discharge of clear yellowish fluid from wound) during follow-up, and group B (10 patients) with an uneventful clinical course (wound healing within 1-2 weeks, with no adjacent tissue response) during follow-up. The control group (group C) was composed of 10 age-matched and practically healthy individuals. Blood samples were taken from the median cubital vein; serum was separated and preserved at -20o C until assays were run. Serum IgM, IgG, IgA and IgE levels were measured using enzyme-linked immunosorbent assays (ELISA) from Xema-Medica Ltd (Russia) and Lab line reader (Austria). Serum circulating immune complexes (CIC) were determined using 3.75% Polyethylene Glycol – 6000 (PEG) serum precipitation, with the light absorbance measured spectrophotometrically on the reader at 450 nm [9]. In addition, serum levels of interleukin (IL)-1? (proinflammatory cytokine), IL-10 (anti-inflammatory cytokine), gamma interferon [TH1(IFN-?)], IL-4 (TH2), and transforming growth factor (TGF)-1? were measured with ELISA kits from Tsitokin Ltd (St Petersburg, Russia) and Vektor-Best (Novosibirsk, Russia). The indirect hemagglutination test as described by Boyden [10] was used to determine the presence of antibodies to protein-polysaccharide complex of human connective tissue antigen [14]. Biostatistika-6 software was used to perform statistical analysis with the non-parametric Wilcoxon U test and Student t-test as per Gubler [7]. Results Table 1 presents peripheral serum levels of IgM, IgG, IgA and IgE in (1) patients with FBT 9 months or later after surgery involving the use of SYNTEKIST biocomposite and (2) practically healthy individuals from the control group. Values for the peripheral serum levels of IgM, IgG, and IgA showed no significant differences (p > 0.05) between both patient groups and the controls. However, the peripheral serum level of IgE in patients from group A (126 IU/mL) was significantly higher (р < 0.05) than in patients from group B (126 IU/mL) and healthy donors.
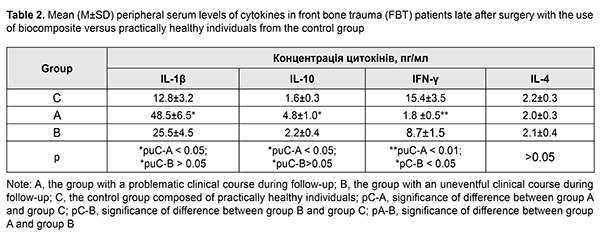
Table 2 presents peripheral serum levels of cytokines IL-1?, IL-10, IFN-?, IL-4 (TH2) in patients and the controls. At the late follow-up point after surgery for FBT, the peripheral serum levels of pro-inflammatory cytokine IL-1? and anti-inflammatory cytokine IL-10 in patients from group A were higher than in patients from group B and donors (Table 2). In patients from group A and those from group B, the serum levels of a key immunity factor, IFN-?, were 2-fold and 8.5-fold, respectively, higher, compared to the controls, whereas those of IL-4, an IFN-? antagonist, were practically the same as in the controls.
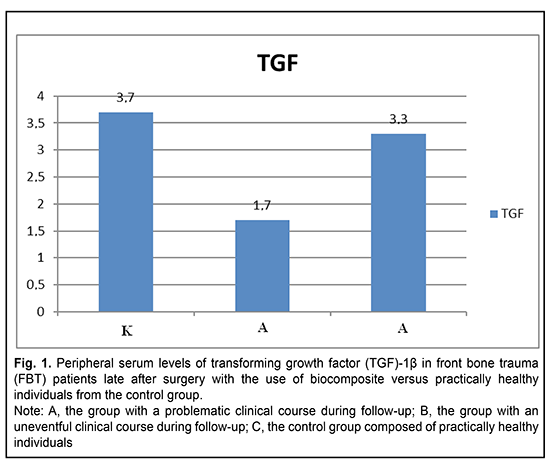
Fig. 1 presents the peripheral serum levels of TGF-1? in patients with FBT late after surgery involving the use of SYNTEKIST biocomposite versus practically healthy individuals from the control group. It can be seen from the figure that, late after surgery with the use of biocomposite for FBT, the peripheral serum level of TGF-1? in patients from group A (1.7 pg/mL) was approximately 2-fold lower than in patients from group B (3.3 pg/mL) and donors (3.7 pg/mL).
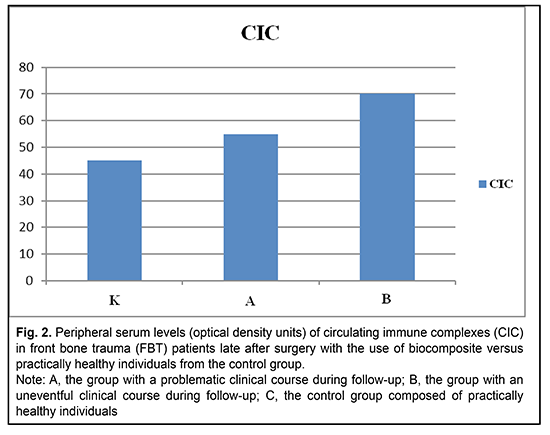
Values for the peripheral serum levels of circulating immune complexes (CIC) showed no significant differences (p > 0.05) between both patient groups and the controls (Fig. 2). Peripheral serum CIC levels in the controls, patients from group A and patients from group B were 45 optical density units (ODU), 55 ODU, and 70 ODU, respectively (p = 0.1).
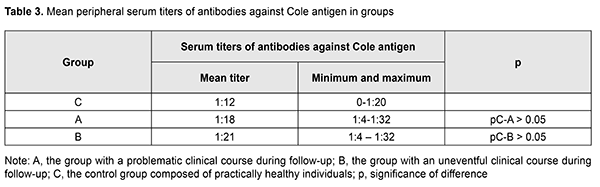
Our study of antibodies to protein-polysaccharide complex of allogeneic connective tissue (Cole antigen) demonstrated no significant difference in antibody levels between patients from group A (with a problematic clinical course), patients from group B and the controls (Table 3).
The above results may point to the absence of autoimmune reaction to connective tissue antigens in patients after surgery involving the use of the biocomposite. Discussion Therefore, we (1) studied FTB patients who exhibited either uneventful or eventful clinical recovery after surgery involving the use of the biocomposite, and (2) found abnormalities in immunity characteristics (like activation of pro- and anti-inflammatory interleukins) in patients exhibiting an eventful recovery; such abnormalities are considered as a manifestation of a prolonged recovery process [3, 11, 13]. Since a significantly decreased blood level of a key immunity factor, IFN-?, has a pathogenetic role [8, 9], the use of interferon inducers as an adjunct to therapy in FBT patients with an eventful recovery might be reasonable. A weakened normalization process in patients with an eventful recovery can be explained also by low levels of growth factors, particularly, TGF-1?, that plays a role in regenerative processes in most tissues of the body. One may think that the absence of autoimmune reaction to connective tissue antigens in all FBT patients after surgery involving the use of the biocomposite reflects the stressful nature of hypoimmune abnormalities. Conclusion First, late after surgery involving the use of the biocomposite, FBT patients with an eventful postoperative course exhibited alterations manifesting as activation of both types of helper cells (increased IL-1 and IL-10 levels). Second, decreased IFN-? and TGF-1? levels in FBT patients with an eventful postoperative course have a pathogenetic role. In addition, measurement of serum IL-1, IL-10, IFN-? and TGF-1? levels may be recommended as objective measures for monitoring rehabilitation process in patients with FBT. References 1.Gorbunov VI, Likhterman LV, Gannushkina IV. [Immunology of traumatic brain injury]. Ulianovsk:SVNTS; 1996. Russian. 2.Lysianyi NI, Pedachenko EG, Kadzhaia NV. [Features of autoimmune responses developing in recurrent TBI]. Immunologiia ta allergologiia. 2006;3:53–6. Ukrainian. 3.Matricon J, Barnish N, Ardid D. [Immunopathogenesis of inflammatory bowel disease]. Med Sci (Paris). 2010 Apr;26(4):405-10. doi: 10.1051/medsci/2010264405. French. 4.Zhuang J, Shackford SR, Schmoker JD, Anderson ML. The association of leukocytes with secondary brain injury. J Trauma. 1993 Sep;35(3):415-22. 5.Kishchuk VV, Bondarchuk OD, Dmytrenko IV, Lobko KA. [Changes in humoral systemic immunity as an objective criterion of the rehabilitation process in patients with frontal basilar trauma]. Zhurnal vushykh, nosovyjh i gorlovykh khvorob. 2018;5:45. Ukrainian. 6.Kishchuk VV, Bondarchuk OD. [Using Syntekist biocomposites to repair bone defects in ear, nose and throat organs]. Zhurnal vushykh, nosovyjh i gorlovykh khvorob. 2007;5c:43–4. Ukrainian. 7.Gubler ЕV. [Informatics in pathology, clinical medicine and pediatrics]. Leningrad:Meditsina; 1990. Russian. 8.Demianov AV, Kotov AIu, Simbirtsev AG. [Diagnostic value of studying cytokine levels in clinical practice]. Cytokines and inflammation. 2003;3:20–8. Russian. 9.Drannik GN. [Clinical immunology and allergology]. Kyiv:Polygraph plus; 2010. Russian. 10.Kabat EA, Mayer MM. [Experimental immunochemistry]. Moscow:Mir; 1968. Russian. 11.Kovalchuk LV, Gankovskaya LV, Meshkova RY. [Clinical immunology and allergology with the basics of general immunology]. Moscow: GEOTAR–Media; 2011. Russian. 12.Nasonov EL. [Methodological aspects of measurements of circulating immune complexes with the use of polyethylene glycol]. Terapevticheskie arkhivy. 1987;4:38–45. Russian. 13.Melnykov OF, Zabolotnyi DI, Maliarenko TV, Prylutska OD. [Rynital in the rational pharmacotherapy of seasonal allergic rhinitis]. Kyiv:Vistka Ltd; 2015. Ukrainian. 14.Iakovenko VD. [Experimental and clinical grounding of the allergic diagnosis of chronical tonsillitis]. [Abstract of Cand Sc (Med) Thesis]. Kyiv: Prof. Kolomiichenko Institute of Otolaryngology; 1985. Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|