J.ophthalmol.(Ukraine).2019;5:27-29.
|
http://doi.org/10.31288/oftalmolzh201952729 Received: 04 September 2019; Published on-line: 30 October 2019 Changes in the expression of molecular markers of peripheral blood lymphocyte activation in patients with small (T1) choroidal melanoma after transpupillary thermotherapy I.V. Tsukanova, Junior Research Associate; S.I. Poliakova, Dr Sc (Med); L.M. Velichko, Dr Sc (Med); O.V. Bogdanova, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) E-mail: inna.sister@gmail.com TO CITE THIS ARTICLE: Tsukanova IV, Poliakova SI, Velichko LM, Bogdanova OV. Changes in the expression of molecular markers of peripheral blood lymphocyte activation in patients with small (T1) choroidal melanoma after transpupillary thermotherapy. J.ophthalmol.(Ukraine).2019;5:27-29. http://doi.org/10.31288/oftalmolzh201952729
Background: Expression of some functional molecules on peripheral blood lymphocytes is critically important for the development of adequate antitumor response. Purpose: To investigate changes in the expression of molecular markers of peripheral blood lymphocyte activation in patients with small (T1) choroidal melanoma (CM; measuring ? 3 mm in protrusion into the vitreous and ? 12 mm in basal dimension) after transpupillary thermotherapy (TTT) delivered using our methodology. Materials and Methods: We investigated the expression of molecular markers (СD7+, CD25+, CD38+, CD45+, CD54+, CD95+, CD150+) of peripheral blood lymphocyte activation using the routine methodology in 16 patients (12 women (75%) and 4 men (25%); mean age, 55.4 years (11.2 years)) with small (T1) CM at baseline and after a course of TTT delivered using our methodology. Statistical analyses were conducted using Statistica 10 (StatSoft, Tulsa, OK, USA) software. Results: Four-day activity of all examined peripheral blood lymphocyte subpopulations in patients was observed in response to the curative effect of 810-nm diode laser TTT on CM. However, statistically significant increases were found only for the expression of the costimulatory molecule inducing cytokine secretion (CD7+) and proaptotic lymphocyte activity due to an increased share of CD95+-phenotype cells. Conclusion: The expression of molecular markers of peripheral blood lymphocyte activation, especially the expression of the costimulatory molecule inducing cytokine secretion (CD7+) and proaptotic lymphocyte activity due to an increased share of CD95+-phenotype cells, increases within the course of TTT for the small (T1) CM. Keywords: choroidal melanoma, transpupillary thermotherapy, molecular markers of peripheral blood lymphocyte activation
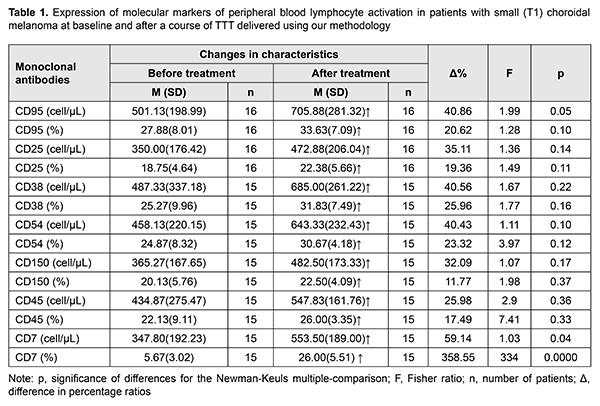
Introduction Much cancer-relating data has been accumulated in recent years evidencing a key role of impairments in immunologic control mechanisms in the initiation and development of the tumor process. Expression of some functional molecules on peripheral blood lymphocytes is critically important for the development of adequate antitumor response [1]. Our previous studies on the expression of molecular markers of peripheral blood lymphocyte activation in patients with the small (T1) choroidal melanoma (CM; measuring ? 3 mm in protrusion into the vitreous and ? 12 mm in basal dimension) demonstrated that, at the initial stage of the tumor (e.g., CM), there was evidence of functional activity of immunocompetent cells in the form of lymphocyte receptor activation to Il-2 (CD25+), increased lymphocyte activation and proliferation (CD38+, CD45+, CD150+) and immunoglobulin production (CD150+), activation of intercellular adhesion (CD54+) and apoptosis (CD 95+), and induction of cytokine secretion (CD7+), these characteristics being significantly higher than in healthy individuals [2]. Therefore, it is important to investigate treatment factors-induced changes in the molecular profile of lymphocytes and the role of some molecules in the implementation of positive curative effect of, particularly, transpupillary thermotherapy (TTT). The purpose of this study was to investigate changes in the expression of molecular markers of peripheral blood lymphocyte activation in patients with small (T1) choroidal melanoma (CM; measuring ? 3 mm in protrusion into the vitreous and ? 12 mm in basal dimension) after TTT delivered using our methodology. Materials and Methods We investigated the expression of molecular markers of peripheral blood lymphocyte activation in 16 patients (12 women (75%) and 4 men (25%); mean age, 55.4 years (11.2 years)) with small (T1) choroidal melanoma at baseline and after a course of TTT delivered using our methodology [3]. The levels of molecular markers of peripheral blood lymphocyte activation were assessed immunohistochemically [4]. Fasting venous blood samples (5 ml) were taken prior to treatment and at day 7 after completion of the treatment course. The immunophenotyping panel of monoclonal antibodies (MCAb) included the antibodies responsive to СD7+, CD25+, CD38+, CD45+, CD54+, CD 95+, CD150+ antigens [5]. Statistical analyses were conducted using Statistica 10 (StatSoft, Tulsa, OK, USA) software. Data are presented as mean (with standard deviation (SD) in parentheses). Univariate analysis of variance (ANOVA) with Fisher’s or Newman-Keuls multiple-comparison was used to evaluate statistical significance of quantitative results. Percentage ratios of lymphocyte activation marker levels and differences in these percentage ratios (?%) were calculated. Results and Discussion Table 1 presents changes in the expression of molecular markers of peripheral blood lymphocyte activation in patients with small (T1) CM after TTT delivered using our methodology.
Four-day activity of all examined peripheral blood lymphocyte subpopulations in patients was observed in response to the curative effect of 810-nm diode laser TTT on CM. Statistically significant increases were found, however, only for the expression of the costimulatory molecule inducing cytokine secretion (CD7+) and proaptotic lymphocyte activity due to an increased share of CD95+-phenotype cells. Although CD7+ marker is an activation (or starting) marker, its expression may decrease with a high activation of regulatory cells (Treg) CD4+ and CD25+ that are important for immune response regulation and may play a key role in the induction of T-cell tolerance toward tumors by inhibiting T cell responses. With a progressive tumor growth, the size of this regulatory lymphocyte subpopulation can increase by 40%, resulting in blockage of the immune response [6-8]. Most activated cells die by apoptosis. FAS-ligand CD95+ plays a role in apoptotic death of activated cells, and consequently, in restraining the immune overresponse. The excess T cells undergo apoptosis after they have performed their function to avoid accumulation of activated cells. Apoptotic lymphocyte death is a very important immune regulation mechanism and means of support for immune homeostasis [6, 7]. In this connection, dynamic monitoring of the expression of molecular markers of peripheral blood lymphocyte activation in patients with the choroidal melanoma within the organ-sparing treatment is important for understanding the development of the tumor process, especially the early stage of the tumor process, and for making a decision on whether it is reasonable to administer immunocorrective therapy. Conclusion The expression of molecular markers of peripheral blood lymphocyte activation, especially the expression of the costimulatory molecule inducing cytokine secretion (CD7+) and proaptotic lymphocyte activity due to an increased share of CD95+-phenotype cells, increases within the course of transpupillary thermotherapy for the small (T1) choroidal melanoma. References 1.Rabson A, Rott IM, Delves PJ. [Really essential medical immunology]. Moscow:Binom; 2006. Russian. 2.Poliakova SI, Velichko LM, Bogdanova OV, Tsukanova IV. Comparison of expression of molecular markers of peripheral blood lymphocyte activation in patients with small uveal melanoma and healthy controls. J Ophthalmol (Ukraine). 2017;1:25-8. Russian. 3.Information Bulletin No. 22, based on Pat. of Ukraine №102,890 issued 25.11.2015. Method for treatment of T1 choroidal melanoma. Authors: Pasyechnikova NV, Naumenko VO, Poliakova SI, Tsukanova IV. Owner: State Institution Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine. Ukrainian. 4.Gluzman DF, Skliarenko LM, Nadgornaia VA, Kriachok IA. [Immunocytochemistry in tumor diagnosis]. Kyiv: Morion;2003. Russian. 5.Velichko LN. [The level of activated lymphocytes molecular markers expression in peripheral blood in patients with uveal melanoma in varying efficiency preserving therapy]. Oftalmol Zh. 2013; 5:9-13. Russian. 6.Berezhnaia NM. [Interleukin system and cancer (new aspects of the interaction between the tumor and the body]. Kyiv:DIA; 2000. Russian. 7.Berezhnaia NM, Chekhun VF. [Immunology of malignant growth]. Kyiv: Naukova Dumka; 2005. Russian. 8.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005 Apr;6(4):345-52.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|