J.ophthalmol.(Ukraine).2019;5:9-17.
|
http://doi.org/10.31288/oftalmolzh20195917 Received: 05 September 2019; Published on-line: 30 October 2019 New risk factors for post-surgical recurrent diabetic maculopathy in type 2 diabetes mellitus S.Yu. Mogilevskyy1, Dr Sc (Med), Prof.; Iu.O. Panchenko2, Cand Sc (Med); S.V. Ziablitsev3, Dr Sc (Med), Prof. 1 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 2 Kyiv Municipal Clinical Hospital “Eye Microsurgery Center”; Kyiv (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: Mogilevskyy SIu, Panchenko IuO, Ziablitsev SV. New risk factors for post-surgical recurrent diabetic maculopathy in type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2019;5:9-17. http://doi.org/10.31288/oftalmolzh20195917
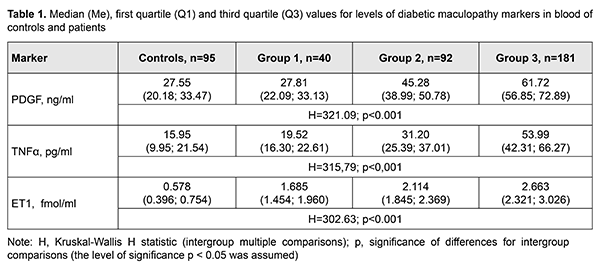
Background: Some pathogenetic risk factors (platelet-derived growth factor (PDGF); tumor necrosis factor alpha (TNF?); and Endothelin-1 (ET1)) are involved in the development of diabetic maculopathy (DMP) in type 2 diabetes mellitus. We hypothesized that the same factors are involved in the formation of postsurgical recurrent DMP. Purpose: To investigate new risk factors of postsurgical recurrent DMP in patients with DM2. Materials and Methods: The study included 313 patients with DM2 (313 eyes) and diabetic maculopathy. These included patients with mild nonproliferative diabetic retinopathy (NPDR; Group 1; n=40), moderate or severe NPDR (Group 2; n=92), and proliferative diabetic retinopathy (PDR; Group 3; n=181). Patients received one of the four types of surgical treatment: only three-port closed subtotal vitrectomy (CSTV; n=78); CSTV combined with internal limiting membrane (ILM) peeling (n=85); CSTV combined with ILM peeling and panretinal laser coagulation (PRLC) (n=81); and CSTV combined with ILM peeling, PRLC and cataract phacoemulsification (phaco) (n=69). Enzyme-linked immunosorbent assay was used to determine presurgical levels of DMP risk factors in blood. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Results: Presurgical levels of DMP risk factors in blood substantially increased with an increase in severity of diabetic retinopathy (from Group 1 to Group 3), with the maximum values achieved in patients with PDR. For each group, PDGF blood levels in patients with recurrent DMP were 1.3- to 1.4-fold (and statistically significantly, p < 0.001) higher than in those without recurrent DMP. TNF? blood levels in Group 1 and Group 2 patients with recurrent DMP were 1.2- to 1.4-fold (and statistically significantly) higher than in those without recurrent DMP. Only for Group 1, median ET1 blood level in patients with recurrent DMP was significantly (1.4-fold; p < 0.001) higher than in those without recurrent DMP. All patients exhibiting recurrent DMP after any of the used surgical treatment technologies had statistically significantly increased baseline PDGF levels (p < 0.001). The pre-surgery cut-off PDGF blood level for which the development of recurrent DMP becomes probable was > 51.8 ng/mL. Associations of TNF? and ЕТ1 blood levels with recurrent DMP were most prominent in NPDR, and depended on recurrence time points: presurgical TNF? blood levels were associated with early recurrent DMP, whereas presurgical ЕТ1 blood levels were associated with late recurrent DMP. Conclusion: New risk factors for post-surgical recurrent DMP in DM2 were established. The PDGF blood level was found to influence the development of both early and late recurrent DMP, whereas the TNF? blood level, only early SMP recurrence, and the ET1 blood level, only late recurrent DMP. Keywords: diabetic maculopathy, surgical treatment, recurrences, PDGF, TNF?, Endothelin-1, type 2 diabetes mellitus Introduction A search is underway to identify predicting and risk factors associated with diabetic maculopathy (DMP) in patients with type 2 diabetes mellitus (DM2) [1, 2, 3]. Duration (more than 6 years) and severity of diabetes (moderate or severe course with transfer to insulin therapy), obesity and arterial hypertension have been found to be among these factors [4]. It is important also to identify pathophysiological factors influencing the development and progression of DMP [5, 6]. Platelet pro-aggregating status triggers thrombosis, retinal vascular hemorrhage, neurosensory and glial cell ischemia, development of inflammation and accumulation of interstitial fluid [6], and seems promising for research. Previously, we have demonstrated the role of platelet proaggregating status in DMP, and the potential for predicting the risk of diabetic retinopathy (DR) and macular edema based on the functional status of platelet receptors [7, 8] An important regulating polypeptide, platelet-derived growth factor (PDGF), is a transmembrane glycoprotein with mitogenic properties [9], and a focus for further research in this area. The major source of PDGF in blood is from platelet alpha-granules, whereas the source in tissues is from fibroblasts, smooth muscle cells and astrocytes [10]. PDGF-ВВ has an important role under conditions of hypoxia and ischemia, when it contributes to endothelial cell proliferation and neoangiogenesis and improves capillary permeability [9, 10]. Retina-specific expression of PDGF-B in transgenic mice has been reported to result in severe neovascularization and retinal detachment, which is typical for ischemic retinopathy [11]. A meta-analysis [12] has shown that, in addition to PDGF, there are number of factors (including tumor necrosis factor alpha (TNF?)) that contribute to the development of DMP. It has been demonstrated that TNF? modulates the effect of insulin on receptors by inducing serine phosphorylation of insulin receptor substrate 1 (IRS-1) at serine residues 636/639 and impeding tyrosine phosphorylation of IRS-1 [13]. This prevents further activation of the PI3K/Akt and ERK/MAP-Kinase Pathways and glucose consumption [14]. Alternative serine/ tyrosine phosphorylation of IRS normally regulates the efficacy of insulin signal transfer, whereas TNF? was shown to stimulate multi-site S/T phosphorylation of IRS1 and IRS2, blocking their interaction with an IR juxtamembrane domain peptide and causing insulin resistance [15]. In our point of view, these mechanisms may also influence the development of recurrent diabetic maculopathy, since they exacerbate DM2 course, especially after surgical procedures that activate proinflammatory cytokine expression by activating Pyrin domain 3 (NLRP3) inflammasomes, the components of pro-inflammatory signalling complexes [16] Currently, the vascular endothelium is recognized as a dynamically regulated organ that plays a key role in various metabolic and vascular disorders including DM2 [4-6]. In DM2, endothelium is a site of a series of pathological reactions to glucotoxicity, intensified oxidative stress, thrombotic activators and excessive action of stimulating hypertensive and inflammatory factors [17, 18]. Endothelial dysfunction emerges in conditions of DM2, is a player in its pathogenesis and causes further development of the disorder [19]. Endothelin-1 (ET1) is a key marker of endothelial dysfunction [17, 20]. Various surgical procedures have been used for the treatment of DMP. They include e.g. closed subtotal vitrectomy (CSTV) that can be combined with internal limiting membrane (ILM) peeling, endolaser coagulation or panretinal laser coagulation (PRLC), and cataract phacoemulsification, if indicated [21]. Favorable treatment outcomes without a substantial risk of decreased visual acuity or other complications have been demonstrated with the use of CSTV combined with cataract extraction in patients with DM2, evidencing high efficacy of the combined surgical intervention [22]. We hypothesized that the pathogenetic factors of the development of DMP in patients with DM2 and diabetic retinopathy in postoperative period play also a major role in recurrent DMP. The purpose of the study was to investigate new risk factors of recurrent diabetic maculopathy after surgical treatment of patients with DM2. Materials and Methods The study included 313 patients with DM2 (313 eyes) and diabetic maculopathy. These included patients with mild nonproliferative diabetic retinopathy (NPDR; Group 1; n=40), moderate or severe NPDR (Group 2; n=92), and proliferative diabetic retinopathy (PDR; Group 3; n=181). Informed consent was obtained from all patients. The study design and protocol were approved by the ethics committee. Each patient underwent an eye examination which included visual acuity assessment, static Humphrey perimetry, refractometry, slit lamp biomicroscopy, gonioscopy, ophthalmoscopy with Volk Super Field lens and Goldmann three-mirror lens (Volk Optical, Mentor, OH) and fundus photography (the ETDRS seven standard fields) and fluoresecent angiography with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). In addition, they underwent spectral domain optical coherence tomography (SD-OCT; Copernicus REVO, Optopol Technology Sp, zo.o, Zawiercie, Poland; scan programs, Retina 3D and Retina Raster) and OCT (Retina Angio mode). We identified the presence of the true decorrelation signal from blood flow within the preretinal vitreous layer in OCTA images for identification of initial vitreoretinal neovascularization and areas of capillary occlusion (ischemia) in the superficial and deep retinal vascular plexuses. Fundus photography (the ETDRS seven standard fields) was performed with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). Fluoresecent angiography (FA) was performed with the fundus camera if (a) mild vitreoretinal neovascularization was suspected but not identified with ophthalmoscopy or fundus photography or (b) the visual function did not correspond either to ophthalmoscopic changes in the macula or OCT findings. Severity of DR and DMP was graded as per the 2002 guidelines of the American Academy of Ophthalmology [21]. Indications for surgical intervention were progressive vision loss; visual field defects in the central and paracentral regions; changes in the quality of vision in the presence of (a) NPDR with refractive macular edema or macular edema with the presence of tangential tractions resulting from incomplete detachment of the posterior hyaloid membrane of the vitreous, (b) PDR with refractive macular edema, presence of epiretinal membranes or presence of tangential or axial retinal tractions and imminent tractional retinal detachment, (c) presence of vitreous, preretinal and/or subhyaloid hemorrhage. Patients received one of the four types of surgical treatment. Seventy-eight patients (78 eyes) had only a three-port 25G+ closed subtotal vitrectomy using the Constellation Vision System with 25+ Total Plus Combined Procedure Pak (Alcon Laboratories, Inc., Fort Worth, TX). Eighty-five patients (85 eyes) had a closed subtotal vitrectomy with ILM peeling at a 2.5-mm- to 3.5-mm macular region without PRLC; ILM peeling was facilitated by staining with Membrane-Blue dye. Eighty-one patients (81 eyes) had a closed subtotal vitrectomy with ILM peeling and panretinal endolaser coagulation. Sixty-nine patients (69 eyes) had cataract phacoemulsification in addition to a closed subtotal vitrectomy, ILM peeling and panretinal endolaser coagulation. Follow-up visits were at months 1, 3, 6 and 12 after surgery. A percentage of post-surgical DMP recurrence after surgery was determined by the presence of macular edema and/or other DMP manifestations (microhemorrhages, hard exudates, etc.) ELISA was used to determine PDGF-BB, TNF? and ЕТ1 levels with Human PDGF-BB Quantikine ELISA Kit (R&D Systems; USA), and kits from Bender Medsystems, and Biomedica Immunoassays (Austria), respectively. Ninety-five age- and sex-matched individuals without ocular pathology or DM2 were used as controls. We investigated possible associations between above factors and early and late recurrent DMP after various types of surgery. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The Kolmogorov-Smirnov and squared chi tests were applied to test for normal distribution of the data. Data are presented as median (Me) (with first quartile (Q1) and third quartile (Q3) in parenthesis) values. Contingency tables were made and non-parametric Pearson chi square test was applied for comparison of categorical variables. The level of significance p ? 0.05 was assumed. Results As distributions of the obtained values were non-normal (Kolmogorov-Smirnov test, р < 0.05; squared chi test, p < 0.001) for all characteristics, non-parametric statistical Kruskal-Wallis and Mann-Whitney tests were applied for multiple and paired comparisons, respectively (Table 1).
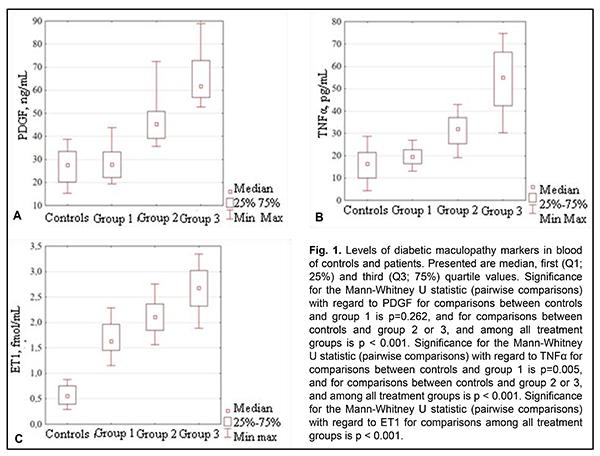
Marker levels in blood of patients were found to increase with increase in severity of DR (Fig. 1). Maximum and minimum PDGF levels were found in patients with PDR and NPDR, respectively, and PDGF blood levels in the former patients (Group 2) were 2.2-fold higher than in controls (p < 0.001). TNF? and ЕТ1 blood levels also were found to increase with increase in severity of DR. TNF? blood levels in patients of group 1, group 2 and group 3 were 1.2-fold (р=0.005), 2.0-fold (p < 0.001) and 3.4-fold (p < 0.001), respectively, higher than in controls. Maximum and minimum TNF? blood levels were found in patients with PDR (Group 3) and mild NPDR (Group 1), respectively. ET1 blood levels in patients of group 1, group 2 and group 3 were 2.9-, 3.7- and 4.6-fold (p < 0.001), respectively, higher than in controls. Maximum and minimum ET1 blood levels were found in patients with PDR (Group 3) and mild NPDR (Group 1), respectively. Table 2 presents levels of DMP markers in blood for patients for patients with the presence or absence of recurrent DMP.
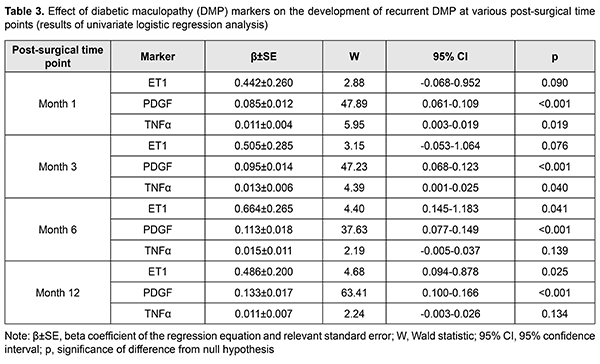
Various types of surgery did not vary in baseline levels of examined markers. For each group, PDGF blood levels in patients with recurrent DMP were 1.3- to 1.4-fold (and statistically significantly, p < 0.001) higher than in those without DMP recurrence. In addition, TNF? blood levels in Group 1 and Group 2 patients with recurrent DMP were 1.2- to 1.4-fold (and statistically significantly) higher than in those without recurrent DMP, which was especially characteristic for patients with mild NPDR. For Group 3, median TNF? blood level in patients without recurrent DMP was higher than in those with recurrent DMP, but with no substantial difference (р = 0.062). Only for Group 1, median ET1 blood level in patients with recurrent DMP was significantly (1.4-fold; p < 0.001) higher than in those without recurrent DMP. There was no significant difference (р>0.2) in ET1 blood level between patients with recurrent DMP and those without recurrent DMP for Groups 2 and 3. For each group, there was no statistical difference (р > 0.1) between various types of surgery with regard to levels of DMP markers in blood. The obtained results determined that there was a need for clarifying the effect of baseline (i.e., pre-surgery) levels of DMP markers in blood on the development of post-surgical recurrent DMP. Univariate logistic regression analysis with the baseline DMP marker blood level for 313 patients involved in the study as an independent variable was used to accomplish this goal. The presence or absence of recurrent DMP (coded as 0 or 1, respectively) was used as a dependent variable. Table 3 presents beta coefficients for the obtained regression equation at various follow-up time points.
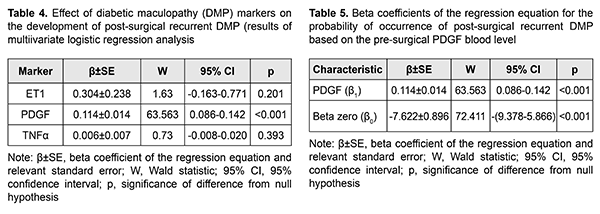
The PDGF blood level was found to have a marked effect on the development of recurrent DMP at all follow-up time points, with w-value varying from 37.63 to 63.41; p < 0.001). The TNF? blood level was found to have an association with the development of recurrent DMP at 1 and 3 months (р=0.019 та р=0.040, respectively). The ET1 blood level (р=0.019 та р=0.040, respectively), but not TNF? blood level was found to have an association with the development of recurrent DMP at 6 and 12 months. Therefore, of the examined DMP factors, the PDGF blood level was found to effect the development of both early and late recurrent DMP, whereas the TNF? blood level, only early recurrent DMP, and the ET1 blood level, only late recurrent DMP. In addition, multivariate logistic regression analysis demonstrated (Table 4) that the PDGF blood level had an effect on the post-surgical recurrent DMP (without adjustments for methods or retinopathy severity).
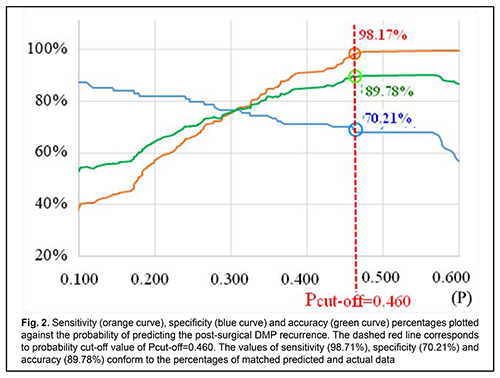
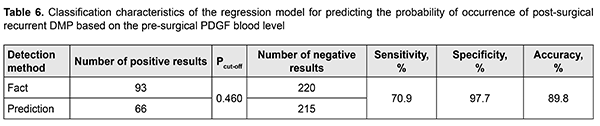
Therefore, we found it reasonable to build a regression model for predicting recurrent DMP based on the baseline PDGF blood level (Table 5). The regression equation for the effect of the baseline PDGF blood level on the probability of recurrent DMP is as follows: P=1/(1+e-7.622+0.114*PDGF) (1), where PDGF is the baseline PDGF blood level. ROC analysis was used to calculate performance measures of the model. Performance measures of the model were satisfactory, with Area Under Curve (AUC) = 0.84; -2*log (Likelihood) = 267.60; ?2 = 114.97 (p<0.001). The curve (Fig. 2) presents the relationship between sensitivity, specificity and accuracy of the built regression model and probability of predicting recurrent DMP by plotting sensitivity, specificity and accuracy against the probability. The curve was used to determine the optimum probability for discriminating between negative and positive predictions. Based on the accuracy characteristic analysis, the probability cut-off was set to Pcut-off=0.460. Table 6 presents classification characteristics of the regression model.
We calculated the pre-surgery cut-off PDGF blood level (in ng/ml) conforming to the development of recurrent DMP to facilitate practical utilization of the obtained data. The following formula was used for this purpose:for the selected Pcut-off value. PDGF=(-ln(1/(Pcut-off)-1)+7.622)/0.114(2), where PDGF is the baseline PDGF blood level and Pcut-off is the probability cut-off value. The pre-surgery cut-off PDGF blood level for which the development of recurrent DMP becomes probable was > 51.8 ng/mL with 89.8% accuracy. Discussion It has been reported [23] that the levels of all PDGF isoforms in vitreous were significantly increased in the PDR group, as compared to controls. In addition, PDGF-AA and PDGF-BB correlated significantly to the severity of PDR. Moreover, PDGF-AB and -BB were significantly lower in vitreous of patients with pre-performed complete panretinal photocoagulation (PRP) as compared to incomplete or without PRP. This is in agreement with our findings of (a) increased PDGF-BB levels in blood of patients with DM2 and DMP, in the presence of mild or severe NPDR, or especially PDR; and (b) association of post-surgical DMP recurrence with baseline PDGF-BB levels in blood. Therefore, first, the fact of increased PDGF blood levels in DM2 patients with DMP in the presence of mild or moderate NPDR was established, and, second, an association of the post-surgery recurrent DMP with baseline PDGF blood levels was established. This was confirmed by regression analysis for all recurrent DMP cases without adjustments for recurrence time point, retinopathy severity and surgical treatment methods. Others [24] have reported that the level of IL-8 in the vitreous body of severe PDR patients undergoing posterior vitrectomy was significantly increased. It has been demonstrated [25] that the levels of pro-inflammatory cytokines in eyes of patients with DR were increased. It is under conditions of PDR that Pyrin domain 3 (NLRP3) inflammasomes, the components of pro-inflammatory signalling complexes [16], mostly become activated. After surgery, the activity of the components of this complex increases, which determined the pathogenetic role of proinflammatory cytokines and their association with recurrent DMP. In addition to angiogenic cytokines, the study by Boss et al [26] found pro-inflammatory cytokines to be important in the development of retinal ischemia under conditions of diabetes mellitus. Therefore, our data are in agreement with those available in the literature (the TNF? level is associated with the severity of retinopathy). However, the association of the factor with the recurrent DMP was not obvious. Thus, for Group 2, the blood cytokine level for the use of all methods of treatment (posterior subtotal vitrectomy, ILM peeling, PRLC and cataract phacoemulsification) was 1.3-fold to 1.4-fold higher than for the use of other methods (р < 0.001). It is likely that a higher intensity of inflammatory response could cause higher expression of pro-inflammatory cytokines [16]. Further, we found that a significant association of the TNF? blood level with the recurrent DMP was in NPDR, but not in PDR. It is likely that for those cases, angiogenic cytokines were more important than pro-inflammatory cytokines, which is in agreement with the opinion of Kovacs et al [26]. This was confirmed also by the results of the regression analysis which demonstrated the association of the TNF? blood level with the early, but not late recurrent DMP. Therefore, there was a significant association of the TNF? blood level with the early but not late recurrent DMP in NPDR. Several studies reported on increased ET1 levels in blood of patients with DT and in vitreous body samples, which was mostly attributed to proliferative effect of ET1. Thus, the formation of epiretinal membranes in PDR is associated with high ET1 levels in tissues and high fibroblast activity [17, 27, 28]. Others have reported that ET1 levels in vitreous body samples were significantly higher than in controls [29]. In addition, ET1 levels correlated with VEGF and TNF? levels not only in vitreous body samples, but also in blood samples. Therefore, a several-fold increased ET1 blood level can be considered as pathogenetic factor for the development of DMP, which confirms that endothelial dysfunction is of key importance for the development of vascular impairments in DM2 [19]. Out data are in agreement with those available in the literature and prove that ET1 and endothelial dysfunction are involved in major pathogenetic mechanisms of DT and associated with its severity. It was demonstrated that, in mild NPDR, the ET1 level was important for the formation of recurrent DMP and especially late recurrent DMP. The absence of probable effect of TNF? and ЕТ1 on the development of recurrent DMP in PDR can be explained by (a) severe damage to the retinal structure in these cases and (b) possible effect of other factors like lL-6, von Willebrand factor (vWf) and sE-selectin [30]. Based on the results of a meta-analysis [12], platelet-derived growth factor BB chain (PDGF-ВВ), transforming growth factor beta (TGF?) and platelet-derived growth factor receptor beta SERPINF1 (PEDF) are other potential candidate factors the development of recurrent DMP in PDR. Therefore, further research is warranted to explore the factors (PDGF, TNF? and ЕТ1) influencing the development of recurrent DMP after surgical treatment of DMP in patients with DM2. Conclusion Baseline levels of examined DMP factors in patients with DM2 were increased, with the most prominent increase related to patients with PDR. All patients exhibiting recurrent DMP after any of the used surgical treatment technologies had statistically significantly increased baseline PDGF levels (p < 0.001). The pre-surgery cut-off PDGF blood level for which the development of recurrent DMP becomes probable was > 51.8 ng/mL with 89.8% accuracy. Associations of TNF? and ЕТ1 blood levels with recurrent DMP were most prominent in NPDR, and depended on recurrence time points: presurgical TNF? blood levels were associated with early recurrent DMP, whereas presurgical ЕТ1 blood levels were associated with late recurrent DMP. References 1.Kamoi K, Takeda K, Hashimoto K, Tanaka R, Okuyama S. Identifying risk factors for clinically significant diabetic macula edema in patients with type 2 diabetes mellitus. Curr Diabetes Rev. 2013 May;9(3):209-17. doi: 10.2174/1573399811309030002. 2.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57(4):347-70. doi: 10.1016/j.survophthal.2012.01.004. 3.Pasyechnikiva NV, Suk SA, Kuznetsova TA, Parkhomenko OG. [Diabetic maculopathy. Current aspects of the pathogenesis, clinical picture, diagnosis and treatment]. Kyiv: Karbon LTD;2010. Russian. 4.Diep TM, Tsui I. Risk factors associated with diabetic macular edema. Diabetes Res Clin Practice. 2013 Jun;100(3):298-305. doi: 10.1016/j.diabres.2013.01.011. 5.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Diabet Stud. 2015 Spring-Summer;12(1-2):159-95. 6.Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology. 2017;24(4):229-41. 7.Mogilevskyy SIu, Panchenko YuO, Ziablitsev SV, Ziablytsev DS. Influence of local and systemic factors of type 2 diabetes mellitus on the functional status of platelets in patients with diabetic and maculopathy. Journal of Ophthalmology (Ukraine). 2018;6(485):23-9. 8.Mogilevskyy SIu, Panchenko IuO, Ziablytsev SV. Predicting the risk of diabetic retinopathy-assosiated macular edema in patients with type 2 diabetes mellitus. Journal of Ophthalmology (Ukraine). 2019;3(488):3-8. 9.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009 Jan;23(1):153-63. 10.Rodriguez A, Friman T, Kowanetz M, van Wieringen T, Gustafsson R, Sundberg C. Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-?1 in normal skin in vivo. Am J Pathol. 2013 Jun;182(6):2132-46. 11.Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001-6. 12.McAuley AK, Sanfilippo PG, Hewitt AW, Liang H, Lamoureux E, Wang JJ, Connell PP. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Complications. 2014 May-Jun;28(3):419-25. 13.IRS1 – Insulin receptor substrate 1 – Homo sapiens (Human) – IRS1 gene & protein". www.uniprot.org. Retrieved 2016-04-21. 14.Takaguri A. [Elucidation of a new mechanism of onset of insulin resistance: effects of statins and tumor necrosis factor-? on insulin signal transduction]. Yakugaku Zasshi. 2018;138(11):1329-1334. 15.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565-82. 16.Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017 Dec;95(8):803-808. 17.Babik B, Pet?k F, Ag?cs S, Blaskovics I, Al?cs E, Bod? K, S?dy R. [Diabetes mellitus: endothelial dysfunction and changes in hemostasis]. OrvHetil. 2018; 159(33):1335-1345. 18.Raminderjit Kaur, Manpreet Kaur, Jatinder Singhcorresponding. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018; 17: 121. 19.Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018; 123 (4): 477-494. 20.Polovina MM, Potpara TS. Endothelial dysfunction in metabolic and vascular disorders. Postgrad Med. 2014; 126(2): 38-53. 21.Balashevich LI, Izmailov AS. [Diabetic ophthalmopathy]. St. Petersburg: Chelovek; 2012. Russian. 22.Xiao K, Dong YC, Xiao XG, Liang SZ, Wang J, Qian C, Wan GM. Effect of pars plana vitrectomy with or without cataract surgery in patients with diabetes: a systematic review and meta-analysis. Diabetes Ther. 2019 Jul 25. 23.Praidou A, Klangas I, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, Dimitrakos S. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009 Feb; 34 (2): 152-61. 24.Raczy?ska D, Lisowska KA, Pietruczuk K, Borucka J, ?lizie? M, Raczy?ska K, Glasner L, Witkowski JM. The level of cytokines in the vitreous body of severe proliferative diabetic retinopathy patients undergoing posterior vitrectomy. Curr Pharm Des. 2018;24(27):3276-3281. 25.Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5594-5603. 26.Kovacs K, Marra KV, Yu G, Wagley S, Ma J, Teague GC, Nandakumar N, Lashkari K, Arroyo JG. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci. 2015 Oct;56(11):6523-30. 27.Blum A, Socea D, Sirchan R. Vascular responsiveness in type 2 diabetes mellitus (T2DM). QJM. 2016; 109(12): 791-796. 28.Chang W, Lajko M, Fawzi AA. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLoS One. 2018 Jan 19;13(1):e0191285. 29.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012 May;37(5):416-20. 30.Adamiec-Mroczek J, Oficjalska-M?y?czak J, Misiuk-Hoj?o M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: Analysis of vitreous samples. Cytokine. 2010 Mar;49(3):269-74. doi: 10.1016/j.cyto.2009.11.004.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|