J.ophthalmol.(Ukraine).2019;5:3-8.
|
http://doi.org/10.31288/oftalmolzh2019538 Received: 09 July 2019; Published on-line: 30 October 2019 Results of using the novel method for predicting the development and course of glaucomatous optic neuropathy in POAG I.V. Yakymenko1, N.A. Ulianova1, Dr Sc (Med), Prof.; L.V. Venger1, Dr Sc (Med), Prof.; K.S. Shakun2, Cand Sc (Phys and Math) 1 Odessa National Medical University; Odesa (Ukraine) 2 Mechnikov Odessa Medical University; Odesa (Ukraine) E-mail: irinapanchak@ukr.net TO CITE THIS ARTICLE: Yakymenko IV, Ulianova NA, Venger LV, Shakun KS. Results of using the novel method for predicting the development and course of glaucomatous optic neuropathy in POAG. J.ophthalmol.(Ukraine).2019;5:3-8. http://doi.org/10.31288/oftalmolzh2019538
Background: Given that the rates of glaucoma-induced visual incapacitation are steadily increasing, predicting glaucomatous optic neuropathy is important. Purpose: To determine the diagnostic importance of the method proposed for the predicting the development and course of glaucomatous optic neuropathy in primary open-angle glaucoma (POAG) based on SweptSource-OCT-derived morphometric characteristics of the lamina cribrosa and daily intraocular pressure (IOP) in the clinical practice. Materials and Methods: Thirty patients were under observation. At baseline, the risk for the development of glaucomatous optic neuropathy in glaucoma suspects or the risk for disease progression in patients with already diagnosed POAG was assessed using our method. The latter is based on the determination of pressure exerted on the ganglion axons at the level of lamina cribrosa, given the specified IOP value and individual morphometric characteristics of the lamina cribrosa. Patients were divided into three groups depending on their risk levels. A DRI OCT Triton plus swept source OCT system (Topcon, Tokyo, Japan) was used to assess morphometric characteristics of the retina (ganglion cell complex thickness) and optic disc (lamina cribrosa thickness and diameter). The further course of the glaucomatous progression was studied using macular GCL++ characteristics. Results: At month 18, GCC thickness loss was statistically significantly larger in the moderate-risk group than in the low-risk group (p=0.001). In addition, it was statistically significantly larger in the high-risk group than in the low-risk group (p=0.00001) and in the moderate-risk group (p=0.00013). In the low-risk group, mean GCC thickness was 92.90±3.28 µm at baseline and 91.95±3.24 µm at month 18; however, the GCC thickness loss was not statistically significant (p=0.838). Conclusion: First, our method for predicting the development and progression of glaucomatous optic neuropathy in POAG based on SS-OCT-derived morphometric characteristics of the lamina cribrosa and daily IOP level enables determining the risk level of glaucomatous progression, which has been evidenced by the results of a prolonged observation. Second, significant macular GCC thinning was noted in 50% of cases of the high-risk group, even with a normal IOP level. Finally, introduction of the proposed method into clinical practice would enable planning effective glaucoma management aimed at prevention of optic nerve atrophy. Keywords: glaucomatous optic neuropathy, predicting, lamina cribrosa, risk level Introduction As primary open-angle glaucoma (POAG) is a multifactorial disease, there are various areas of research related to predicting the course of the disease. A method for calculating both the index of volumetric blood flow and the ratio of blood flow velocities of superior ophthalmic vein and internal carotid artery has been proposed on the basis of the vascular theory of POAG [1]. Given the role of free radical oxidation in the development of POAG, levels of peroxidation products in the anterior chamber aqueous humor, a potential factor for destruction of anterior eye tissue, have been investigated, allowing for identification of susceptibility to glaucomatous optic neuropathy [2, 3]. Studies on genetic susceptibility to POAG represent a major area of research related to predicting the course of the disease [4, 5]. Intraocular pressure (IOP) is the best studied factor of the development of POAG. The concept of “target IOP” is used to determine the IOP reduction. According to the European Glaucoma Society Terminology and Guidelines for Glaucoma, in general, when there is more advanced damage, lower IOPs are needed to prevent further progression [6], which is pathogenetically substantiated. As the development of glaucomatous optic neuropathy is the main cause of decreased visual function POAG, currently, POAG is defined as a chronic neurodegenerative disease developing due to degeneration of retinal ganglion cells and their axons, and leading to thinning of peripapillary retinal nerve fiber layer, increased optic disc excavation, and visual function loss [7]. Optical coherence tomography (OCT) is the gold standard for diagnosing POAG and determining the severity of glaucomatous optic neuropathy; particularly, morphometric analysis of the macula and optic disc includes determination of retinal nerve fiber layer (RNFL) thickness, retinal ganglion layer thickness, neuroretinal rim volume, excavation area, excavation volume, cup/disc ratio and disc damage likelihood scale (DDLS). It is known that structural changes can be revealed based on OCT long before abnormal functional manifestations occur [8]. Although current diagnostic potential for early detection of glaucomatous optic neuropathy is rather high, the rates of visual incapacitation due to POAG-related irreversible loss of sight are steadily increasing [9]. Frequency doubling technology (FDT) perimetry with the Humphrey Matrix (Carl Zeiss Meditec, Inc., Dublin, CA) has been widely used for predicting the course of POAG. Visual field changes can be predicted using FDT after static perimetry (SAP). Although the capacity for prediction of visual field loss among glaucoma suspect patients is an advantage of FDT perimetry, the results of this method may be influenced by a number of external factors like patient’s delayed response, general and neurological condition, and presence of concomitant eye disease. However, given that functional changes in glaucoma are preceded by structural changes in the retina and optic nerve, we believe that the methods based on the analysis of preclinical structural changes in the retina and optic nerve are more relevant to the prediction of the development of POAG. Therefore, the issue of predicting the onset and progression of glaucomatous optic neuropathy is important. The purpose of this study was to determine the diagnostic importance of the method proposed for the predicting the development and course of glaucomatous optic neuropathy in POAG based on SweptSource-OCT morphometry study of the lamina cribrosa and daily IOP measurement in the clinical practice. Materials and Methods Thirty patients (14 women (46.7%) and 16 men (53.3%); age, 56 to 78 years; mean age, 65.6±1.06 years) were under observation. All these underwent a routine eye examination. A DRI OCT Triton plus swept source OCT system (Topcon, Tokyo, Japan) was used to perform morphometry of the retina and optic disc. Radial 9 x 9 mode was used in the optic disc study for better visualization of the lamina cribrosa (LC). Lamina cribrosa diameter and thickness were measured. 3D Macula mode was used to perform macular study. In the current study, we used the method that we proposed and mathematically substantiated in [10, 11] to assess either the risk for the development of glaucomatous optic neuropathy in glaucoma suspects or the risk for disease progression in patients with already diagnosed POAG. Under conditions of increased IOP, optic nerve fibers become compressed at the level of lamina cribrosa due to longitudinal and transverse displacement of its layers. The method is based on determining the pressure (P) exerted on the ganglion axons at the level of lamina cribrosa, given the specified IOP value and individual morphometric parameters of the lamina cribrosa. The following formula for determination of pressure exerted on the ganglion axons at the level of LC was derived after physical and mathematical LC modeling and a series of calculations:
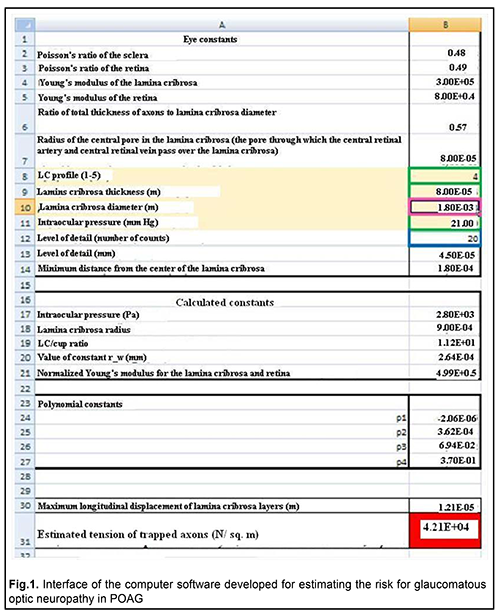
Where P, pressure exerted on the ganglion axons; ur, longitudinal displacement of LC layers at distance ri from the center of LC; d, aggregate thickness of the axonal fibers crossing the LC; ?, normalized Young’s modulus; rw*, modeling parameter; ? (h), function mapping the LC thickness seen on OCT images to the actual LC thickness; h, LC thickness seen on OCT images The risk for the development or progression of glaucomatous optic neuropathy could be assigned three levels depending on the P value with respect to retinal yield (p0), a physical constant equal to 9 kPa. We designed computer software for automatically determining the risk level (Fig. 1). The risk is absent if Р?р0, and moderate if р0?Р?3?р0. The risk for the development or progression of glaucomatous optic neuropathy is high if Р?3?р0 and irreversible loss of nerve filaments occurs.
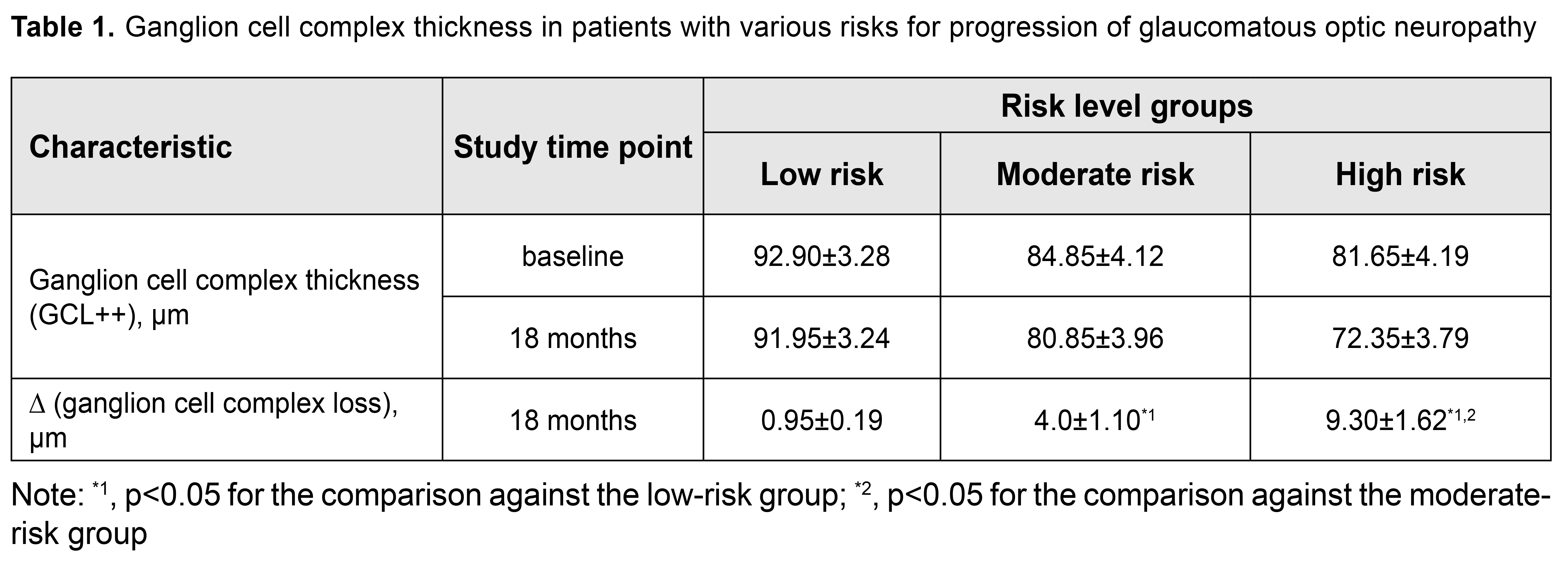
Our patients were divided into three groups depending on their risk levels. Group 1 included 10 patients (20 eyes) with low risk, Group 2 included 10 patients (20 eyes) with moderate risk, and Group 3 included 10 patients (20 eyes) with high risk for the development or progression of glaucomatous optic neuropathy. Interestingly, each group included patients with grade 1, 2 and 3 POAG as well as glaucoma suspects. In each patient both eyes had equal risk levels. Exclusion criteria were terminal glaucoma and concomitant eye disease. At baseline, the risk for the development or progression of glaucomatous optic neuropathy was determined for each patient. Ganglion cell complex (GCC) includes the inner plexiform layer (IPL), retinal nerve fiber layer (RNFL), and retinal ganglion cell layer (RGCL); given a high informative value of OCT-based GCC data in the diagnosis of glaucoma [12, 13], the further course of the progression of glaucomatous optic neuropathy was studied using macular GCL++ characteristics. The total observation period was 18 months. All groups were comparable with regard to tonometric characteristics. It is noteworthy that IOP was normal in all cases, with the mean values of 18.75±0.54 mmHg, 19.4±0.34 mmHg and 19.65±0.41 mm Hg for the low-risk, moderate-risk and high-risk groups, respectively. Tonometric characteristics were assessed as average daily corneal thickness-adjusted IOP measured by the Maklakov tonometer [14]. The results were assessed by calculating means of variational series (M) with standard error of the mean (m). In addition, Student’s test was used to determine significance. The level of significance p ? 0.05 was assumed. Results and Discussion In the low-risk group, mean GCC thickness was 92.90±3.28 µm at baseline and 91.95±3.24 µm at month 18; however, the GCC thickness loss was not statistically significant (p=0.838). The same tendency was observed for the moderate-risk and high-risk groups. That is, the intra-group difference was not statistically significant for all the three groups. However, it is noteworthy that there were significant statistical differences between the groups with regard to GCC thickness loss (Table 1). Thus, GCC thickness loss was statistically significantly larger in the moderate-risk group than in the low-risk group (p=0.001). In addition, it was statistically significantly larger in the high-risk group than in the low-risk group (p=0.00001) and in the moderate-risk group (p=0.00013). Noticeable GCC loss was found in 20% of cases of the moderate-risk group. Significant thinning of GCC layers was noted in 50% of cases of the high-risk group.
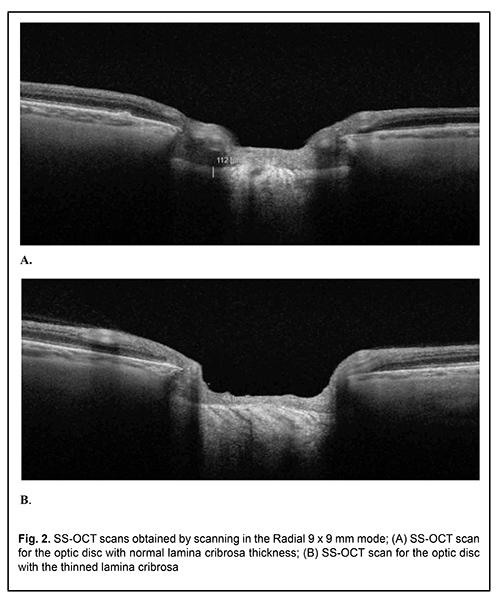
Our glaucoma prediction model has been based on the mathematically proved relationship between the individual morphological parameters of LC and IOP level. The lamina cribrosa was given an important role in the development of glaucomatous optic neuropathy as early as nineteen seventies and eighties [15]. Deformation of the lamina cribrosa is known to result in displacement and compression of and damage to the axons of the retinal ganglion cells and vessels that penetrate it. This leads to pathological processes (like ischemia, free radical production, apoptosis, and metalloprotease activation) exerting a cytotoxic effect on retinal and optic nerve cells. With the advent of advanced technologies enabling visualization and accurate assessment of the condition of LP, studies in this area have become increasingly popular. Thus, it has been reported that progression of glaucoma is accompanied by lamina cribrosa thinning [16, 17, 18], which can be followed up with OCT (Fig. 2).
It is thinning and IOP-induced deformation of the LP that are a key stage in the development of glaucomatous optic neuropathy. Our method for predicting the development and progression of POAG has been based on this morphofunctional relationship. Therefore, the amount of balance between morphometric characteristics of the LP and IOP influences LP structure and determines the risk of disease development. The pilot studies demonstrated that our method was highly efficient. Technically substandard LC visualization is, however, possible with OCT machines of previous generations, which is a limitation of the diagnosis method. Conclusion First, our method for predicting the development and progression of glaucomatous optic neuropathy in POAG based on SS-OCT-derived morphometric characteristics of the lamina cribrosa and daily IOP level enables determining the risk level of glaucomatous progression, which has been evidenced by the results of a prolonged observation. Second, significant macular GCC thinning was noted in 50% of cases of the high-risk group, even with a normal IOP level. Finally, introduction of the proposed method into clinical practice would enable planning effective glaucoma management aimed at prevention of optic nerve atrophy. References 1.Chinarev VA, Galiakberova ZR. [Predicting progression of primary open-angle glaucoma]. Bulletin of the Council of Young Scientists and Specialists of the Chelyabinsk Region. 2016;3 (14):99–101. Russian. 2.Bunin AY. [Metabolic factors of the pathogenesis of primary open – angle glaucoma]. In: [Glaucoma at the turn of the millennia: results and prospects. Proceedings of the Russian National Science Conference]. Moscow, 1999. Russian. 3.Soliannikova OV, Berdnikova EV, Ekgart VF. [Predicting the time course of visual functions in patients with primary open–angle glaucoma]. Ophthalmology Journal. 2015; 8 (1):36–42. Russian. 4.Wang R, Wiggs JL. Common and rare genetic risk factors for glaucoma. Cold Spring Harb Perspect Med. 2014, Sep. 18; 4(12): a017244. doi: 10.1101/cshperspect.a017244. 5.Sergienko AM, Melnik VO, Khoroshkova MV. [Genetic susceptibility to the development of primary open-angle glaucoma]. J Ophthalmol (Ukraine).2018;6:71-5. Ukrainian. 6.European Glaucoma Society. Terminology and Guidance. 4th ed. 2017. 7.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14; 311(18):1901–11. 8.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29(4):249 –71. 9.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta–analysis. Ophthalmology. 2014; 121(11): 2081–90. 10.Patent of Ukraine for utility model UA 133897 U, А61В 8/10 (2006.01). [Method for predicting the development and course of glaucomatous optic neuropathy]. Iakymenko IV, Ulianova NA, Shakun KS. No. 133897; stated. 11.23.2018; published 04.25.2014. Bul. No.8. Ukranian. 11.Iakymenko IV, Ulyanova NA, Shakun KS. [Predicting the development of glaucomatous optic neuropathy. Part I. Mathematical model of lamina cribrosa deformation and damage to nerve filaments in glaucoma]. Oftalmologiia. Vostochnaia Evropa. 2018;4(8):84-92. Russian. 12.Na JH, Lee K, Lee JR, Baek S, Yoo SJ, Kook MS. Detection of macular ganglion cell loss in preperimetric glaucoma patients with localized retinal nerve fibre defects by spectral-domain optical coherence tomography. Clin Exp Ophthalmol. 2013 Dec;41(9):870-80. doi: 10.1111/ceo.12142. 13.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014 Jul;98 Suppl 2:ii15-9. doi: 10.1136/bjophthalmol-2013-304326. 14.Kohlhaas M, Boehm AG, Spoerl E, et al. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 2006 Apr;124(4):471-6. 15.Nesterov AP. [Glaucoma]. Moscow:Medinformagenstvo; 2008. Russian. 16.Chung HS, Sung KR, Lee JY, Na JH. Lamina Cribrosa-Related Parameters Assessed by Optical Coherence Tomography for Prediction of Future Glaucoma Progression. Curr Eye Res. 2016 Jun;41(6):806-13. 17.Lee EJ, Kim TW, Kim M, Kim H. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology. 2015 Apr;122(4):721-9. 18.Omodaka K, Takahashi S, Matsumoto A, et al. Clinical factors associated with lamina cribrosa thickness in patients with glaucoma, as measured with Swept Source Optical Coherence Tomography.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|