J.ophthalmol.(Ukraine).2019;4:33-37.
|
http://doi.org/10.31288/oftalmolzh201943337 Retinal structural changes in patients with Alzheimer's disease R.N. Guliyeva, Junior Research Associate Acad. Z. Aliyeva National Ophthalmology Center; Baku (Azerbaijan) TO CITE THIS ARTICLE: Guliyeva RN. Retinal structural changes in patients with Alzheimer's disease. J.ophthalmol.(Ukraine).2019;4:33-37. http://doi.org/10.31288/oftalmolzh201943337
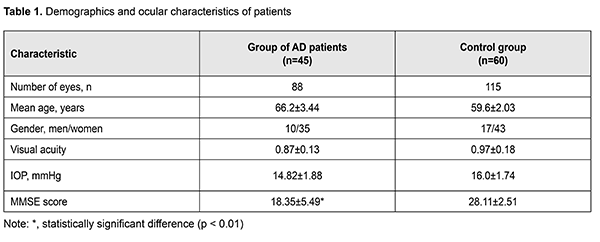
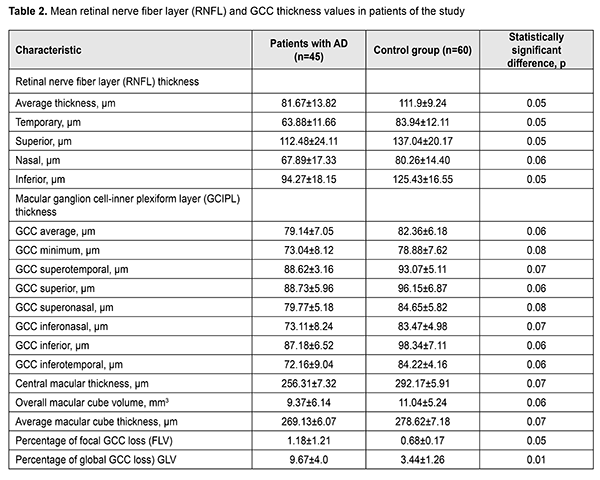
Background: Retinal disorders in patients with Alzheimer's disease (AD) are given special attention. Purpose: To identify optical coherence tomography (OCT)-based retinal structural changes in patients with mild or moderate AD. Materials and Methods: Forty-five AD patients (88 eyes; age, 66.2 ± 3.44 years) with mild or moderate cognitive impairments were involved into the study. All subjects underwent a conventional eye examination and OCT measurements. For Cirrus HD-OCT imaging, the Macular Cube 512 ? 128 Combo protocol was used. The RNFL, retinal ganglion cell layer (RGCL), and internal plexiform layer (IPL) thicknesses were determined using RNFL and ganglion cell complex (GCC) protocols. Results: Compared with controls, patients with AD had 53.2% lower Mini Mental State Examination (MMSE) scores (p < 0.05). In addition, RNFL thicknesses in the temporal, superior and inferior quadrants in patients with AD were significantly (31.4%, 21.8% and 33.1%, respectively; p < 0.05) thinner compared to controls. Moreover, compared to controls, focal loss volume (FLV) and global loss volume (GLV) of GCC in AD patients were 1.7 times (p < 0.05) and 2.8 times (p < 0.01), respectively, increased, whereas central macular thickness, overall macular cube volume and average macular cube thickness were 14%, 17.8% and 3.5% decreased. Conclusion: Our findings confirm the reports of changes in the retinal structure in patients with mild or moderate AD, particularly, the decreased GCC thickness and increased GCC FLV and GLV. Keywords: retina, Alzheimer's disease, RNFL, thickness, GCC, macular cube
Introduction Alzheimer's disease (AD) is a common neurodegenerative disorder affecting more than 10% of persons age 65 years or older. AD is the fifth leading cause of death in people age 65 years or older [1, 2]. According to the World Alzheimer Report of 2016, it is estimated that 47 million people suffer from dementia worldwide, and this number could increase to more than 131 million by 2050, as population ages. AD accounts for 60% to 70% of all dementias [3, 4, 5]. Life expectancy has increased dramatically over the past century across the globe and consequently has been detected an increase in the observed prevalence of chronic diseases among the elderly people [5]. Diagnosis of AD is a certain challenge to the doctor. Alzheimer's disease may begin years before the onset of clinical symptoms due to a slow progression of neurodegenerative processes. Various visual disturbances may affect AD patients, which have been historically attributed to damage and/or degenerative processes in primary and associative visual cortical areas [6]. However, during the last few decades, some authors realized that cortical dysfunctions alone cannot explain entirely the pattern of observed defects [7]. Specifically, multiple forms of evidence points toward the involvement of retinal ganglion cells and their axons in the optic nerve as a basis of the visual dysfunction in AD. In fact, histopathological lesions associated with AD (neuronal loss, beta-amyloid plaques, neurofibrillary tangles, and granulovacuolar degeneration) have been seen not only in brain structures historically thought to be involved in AD, but also within the neuroretina [8, 9]. Various anterior and posterior eye structures are involved in ophthalmic changes associated with AD. In the posterior pole, retinal thickness is correlated to cortical atrophy and choroid and retinal nerve fiber layer (RNFL) is thinning in AD patients [2]. Human and animal studies demonstrated that activated microglia and proin?ammatory molecules are contributing to the development of the pathology [10]. Visual alterations in AD became the focus of a discussion among medical professionals, directing many researchers towards the discovery and development of novel ocular biomarkers and diagnostic tools. The purpose of the study was to identify optical coherence tomography (OCT)-based retinal structural changes in patients with mild or moderate AD. Materials and Methods Forty-five AD patients (88 eyes; age, 58 to 74 years) with mild or moderate cognitive impairments (Mini Mental State Examination (MMSE) score of 13 to 24) were involved into the study. The inclusion criteria were having normotensive eyes and ability to understand the purpose of the study. Patients with a refractive error > 5 D or axial length > 25 mm in the examined eye, severe dementia or other types of dementia (like cerebrovascular, metabolic or psychic disorders), severe ocular disorder (glaucoma, uveitis, or retinal disorder), diabetes or history of stroke were excluded from the AD group. The control group included 60 patients (115 eyes; age, 57 to 68 eyes) without AD and severe ocular disease. Informed consent was obtained, and all procedures followed were in accordance with the Helsinki declaration [11]. Patients underwent Snellen visual acuity testing and Goldman intraocular pressure (IOP) measurements. The MMSE was used for cognitive assessment [12]. In addition, patients underwent OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA). For Cirrus HD-OCT imaging, the Macular Cube 512 ? 128 Combo protocol was used. The RNFL, retinal ganglion cell layer (RGCL), and internal plexiform layer (IPL) thicknesses were determined using RNFL and ganglion cell complex (GCC) protocols. Macular cube scans of an area of 6 x 6 mm2 were centered on the fovea using the Macular Cube 512 ? 128 Combo protocol. The built-in software was used to produce retinal thickness maps, which then were averaged over nine retinal subfields in a 6 mm diameter circle centered at the true fovea location, as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS). The ETDRS areas included a central 1 mm disc and inner and outer rings of 3 and 6 mm, respectively. The central foveal subfield thickness (i.e., central macular thickness) bounded by the innermost 1 mm diameter circle was calculated. The overall average macular thickness (cube average thickness) and overall macular cube volume over the entire grid area were also obtained from the computational software output based on the proportional contribution of the regional macular thicknesses. The Ganglion Cell Analysis algorithm processes data from 3-dimensional volume scans using the macular 512 x 128 acquisition protocol. The algorithm identifies the outer boundary of the RNFL and the outer boundary of the IPL. The difference between the RNFL and the IPL outer boundary segmentations yields the combined thickness of the RGC layer and the IPL. It provides a measurement of the macular GCIPL thickness within a 14.13-mm2 elliptical annulus area centered on the fovea. The mean, minimum, and 6individual sector (superior, superonasal, inferonasal, inferior, inferotemporal, and superotemporal) GCIPL thicknesses were provided. The minimum GCIPL measurement is determined by sampling 360 measurement spokes extending from the center of the fovea to the edge of the ellipse in 1-degree intervals and selecting the spoke with the lowest average [13]. Statistical analyses were conducted using Microsoft Excel 2016 and Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Data are presented as mean ± standard deviation (SD). The level of significance p ? 0.05 was assumed. Results There was no significant difference between the groups with regard to age, gender or visual acuity (Table 1). Patients with AD exhibited significantly (53.2%; p < 0.01) lower MMSE score compared to controls (Table 1).
Retinal and GCC thickness values are presented in Table 2. The average RNFL thickness in patients with AD was significantly (37.0%) lower than in controls. In addition, RNFL thicknesses in the temporal, superior and inferior quadrants in patients with AD were significantly (31.4%, 21.8% and 33.1%, respectively) thinner compared to controls. Moreover, RNFL thickness in the nasal quadrant in patients with AD was 14.2% thinner compared to controls.
The average, minimum, superotemporal, superior, superonasal, inferonasal, inferior and inferotemporal GCC thicknesses in patients with AD were 4.1%, 8.0%, 5.0%, 8.4%, 6.1%, 14.2%, 12.8% and 16.7% thinner compared to controls, but this differences were not significant. Compared to controls, focal loss volume (FLV) and global loss volume (GLV) of GCC in AD patients were 1.7 times (p < 0.05) and 2.8 times (p < 0.01), respectively, increased, whereas central macular thickness, overall macular cube volume and average macular cube thickness were 14%, 17.8% and 3.5% decreased. Therefore, patients with AD exhibited retinal structural changes compared to controls. Discussion We presented the results of the retinal study in patients with mild or moderate Alzheimer's disease. Compared to controls, OCT-based peripapillary RNFL thickness was significantly (p < 0.05) decreased. In addition, in these patients, a decrease in RNFL thickness was significant in the temporal, superior and inferior quadrants, but not in the nasal quadrant. Our findings with regard to a decreased RNFL thickness in the temporal, superior and inferior quadrants in patients with AD are in agreement with those reported by others [14,15]. With regard to a decreased RNFL thickness in the nasal quadrant in patients with AD, our findings are in agreement with those reported by Berisha et al [16], who found no significant difference in RNFL thickness in the nasal quadrant between patients with mild or moderate AD and controls. However, our findings with regard to a decreased RNFL thickness in the inferior and temporal quadrants in patients with AD are in disagreement with their findings. Berisha et al [16] found a decreased RNFL thickness in the superior, but not in the inferior or temporal quadrants [16]. Lu et al [17] also observed a significantly decreased RNFL thickness in the superior and inferior quadrants in patients with AD. They found a significantly decreased OCT-based RNFL thickness in the superior and inferior quadrants in patients with mild or moderate AD compared to controls. In addition, they [17] found that the OCT-based RNFL thickness in the nasal quadrant in these patients was decreased, but not significantly. Early detection of AD is of primary importance. Easy retinal imaging provides for using the retina as for the development of neurodegenerative disorders. Advances in imaging (particularly, OCT imaging) of the eye have allowed for imaging of and quantitative assessment for individual retinal layers. The current study demonstrated that macular changes reflect neurodegenerative changes in AD. In addition, compared to controls, RGCL and IPL average and minimum thicknesses, thickness in the superotemporal, superonasal, inferonasal, inferior and inferoremporal segments of the RGCL and IPL in patients with AD were decreased, but the differences were not significant. Our findings are in agreement with those reported by several studies [18, 19,20]. Conclusion Our findings confirm the reports of changes in the retinal structure in patients with mild or moderate AD, particularly, the decreased GCC thickness and increased GCC FLV and GLV. Further research is warranted to identify retinal biomarkers in AD.
References 1.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2013;9(2):208–45. 2.Colligris P, Perez de Lara MJ, Colligris B, Pintor J. Ocular Manifestations of Alzheimer's and Other Neurodegenerative Diseases: The Prospect of the Eye as a Tool for the Early Diagnosis of Alzheimer's Disease. J Ophthalmol. 2018;2018: 8538573. 3.Alzheimer's Disease International World Alzheimer Report 2016: Improving healthcare for people living with dementia. London; 2016. 4.WHO. Dementia: a Public Health Priority. World Health Organization; 2012. 5.Trebbastoni A, D’Antonio F, Bruscolini A, Marcelli M, Cecere M, Campanelli A, et al. Retinal nerve ?bre layer thickness changes in Alzheimer’s disease: results from a 12-month prospective case series. Neurosci Lett. 2016;629:165–70. 6.Coppola G, Di Renzo A, Ziccardi L, Martelli F, Fadda A, Manni G. et al. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One. 2015;10(8):e0134750. 7.Armstrong RA. Alzheimer's Disease and the Eye. J Optom. 2009;2(3):101-58. 8.Wu Y, Wang X, Wang N, Han Y, Lu Y. Observation Study of the Retina with the Alzheimer’s Disease or Amnestic Mild Cognitive Impairment Patients. J Clin Exp Ophthalmol. 2016;7(2):545. 9.Lim JKH, Li Q-X, He Z, Vingrys AJ, Wong VHY, Currier N, Mullen J, Bui BV, Nguyen ChTO. The Eye As a Biomarker for Alzheimer's Disease. Front Neurosci. 2016;10:536–549. 10.Ramirez AI, de Hoz R, Salobrar-Garcia E, Salazar JJ, Rojas B, Ajoy D. et al. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucoma. Front Aging Neurosci. 2017 Jul 6;9:214. 11.The World Medical Association (WMA) Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects Adopted by the General Assembly, Seoul, Korea, October 2008. 12.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psych Res. 1975; 12:189-98. 13.Mwanza JC, Durbin MK, Budenz DL, Sayyad FE, Chang RT, Neelakantan A, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119(6):1151–8. 14.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112:1860–1867. 15.Iseri PK, Altinas T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. 16.Berisha F, Feke GT, Trempe CL, Mcmeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285-9. 17.Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D. Retinal nerve fiber layer abnormalities in early Alzheimer’s disease: Evidence in optical coherence tomography. Neurosci Lett. 2010. 9;480(1):69-72. 18.Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(9):5953–5958. 19.Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2015;45(1):45–56. 20.Choi SH, Park SJ, Kim NR. Macular Ganglion Cell-Inner Plexiform Layer Thickness Is Associated with Clinical Progression in Mild Cognitive Impairment and Alzheimers Disease. Plos One. 2016;11(9): e0162202. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|