J.ophthalmol.(Ukraine).2019;4:18-22.
|
http://doi.org/10.31288/oftalmolzh201941822 Mistakes in the treatment of pterygium G. I. Drozhzhina, Dr Sc (Med), Prof.; L.F. Troichenko, Cand Sc (Med); V.L. Ostashevskii, Cand Sc (Med); B.M. Kogan, Cand Sc (Med); T.B. Gaidamaka, Dr Sc (Med); E.V. Ivanovskaia, Cand Sc (Med) SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) E-mail: tlf2008@ukr.net TO CITE THIS ARTICLE: Drozhzhina G I, Troichenko LF, Ostashevskii VL, Kogan BM, Gaidamaka TB, Ivanovskaia EV. Mistakes in the treatment of pterygium. J.ophthalmol.(Ukraine).2019;4:18-22.http://doi.org/10.31288/oftalmolzh201941822
Abrupt thinning and even perforation at the limbus and cornea due to deep pterygium excision are potential postoperative complications of pterygium syrgery. Postoperative complications were observed in 25.7% of patients after primary pterygium excision. The analysis of mistakes made in the surgical treatment of pterygium enabled the following recommendations to be made. First, pterygium should be excised with minimal trauma to the limbus and cornea. Second, the patient should be promptly referred to the medical facility at which he/she can undergo keratoplasty if complications (perforations and/or ulcers) develop during pterygium excision. Third, a patient’s concomitant autoimmune disease should be taken into account. Fourth, one should not perform bilateral pterygium surgery or repeat pterygium excision early (? 6 months) after primary pterygium surgery. Finally, steroid and antimetabolic therapy, barrier and optic lamellar keratoplasty, and amniotic membrane transplantation should be used to prevent pterygium recurrence. Keywords: pterygium, treatment, mistakes
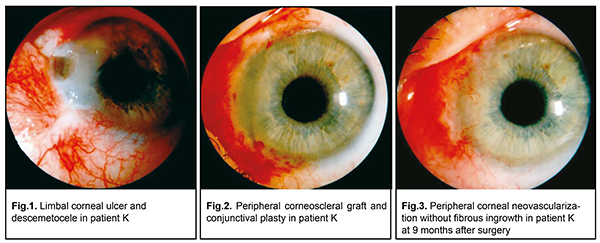
A pterygium is a benign fibrovascular subepithelial ingrowth of conjunctival tissue over the limbus onto the cornea due to limbal epithelial cell hyperproliferation, which is accompanied by neovascularization [1]. It is most common in individuals of working age (30-35 years) [2]. The prevalence is 3% to 20%, being especially high in countries with hot climate and high insolation [2-5]. The disease has been treated over three thousand years. Most authors believe that pterygium management boils down to pterygium excision. Any conservative pterygium management is symptomatic and temporary. Neither of the available surgical techniques for pterygium can completely exclude the possibility of postoperative complications [1, 6-8]. The major goal of pterygium surgery is to completely excise the head, neck and body of the pterygium. There are at least two major approaches to achieving this goal. The first approach involves the following. After adequate topical anesthesia, the head of the pterygium is grasped with forceps, and a surgical blade is used to excise it from the cornea. Thereafter, the pterygium neck and body are dissected with Wescott scissors posteriorly, approximately 4-6 mm from the limbus, and removed. The second approach is to use scissors to undermine and dissect the pterygium body inferiorly. A blunt-edged instrument is then inserted under the body of the pterygium and while pulling on the body with forceps to form a tent; the head is sliced off the cornea by a sawing motion of the iris repositor (avulsion technique). Any pterygium tissue remnants on the cornea are gently scraped off with the surgical blade until a clear corneal bed is obtained. The bed is encouraged to achieve adequate hemostasis. Argon laser and the excimer “laser blade” have been used in pterygium excision to make the corneal surface smooth [1]. In addition, high-frequency welding of biological tissues with a high-frequency current generator EK-300M1 has been used to fix a free limbal conjunctival autograft [9]. Outcomes of operative treatment for pterygium are affected by such postoperative complications as inadequate conjunctival adaptation at the site of excised pterygium; postoperative astigmatism and pterygium recurrence [1, 10]. Until now, there is no consensus on the definition of pterigium recurrence. However, there is a common opinion that pterigium recurrence occurs when there is a fibrovascular ingrowth at the corneolimbal interface. Pterigium recurrence commonly occurs within a year (most commonly, within 6 months) after pterygium surgery, and is seen in more than 40%-70% cases [1, 11]. The proper choice of the surgical technique to be employed as well as adequate postoperative treatment is the mainstay for the prevention of pterigium recurrence. By adequate postoperative treatment of pterigium we understand the use of X-ray of beta-ray brachytherapy; photodynamic therapy (PDT); topical chemotherapeutic antirumoer agents, cytostatic agents (mitomycin C, thiophosphamidum, fluorouracil, and 5-fluorouracil) [12-15]. Current surgery for pterygium is based on meticulous removal of altered conjunctival and subconjunctival tissue and subsequent defect closure with a conjunctival flap taken from the affected or fellow eye. Operative treatment for pterygium should be aimed not only at performing conjunctival plasty, but also at the best possible improvement in corneal transparency and avascularization. A so called barrier approach is preferred for recurrent pterygium, with transplantation of biocompatible tissues using such techniques as autolimbal grafting; Filatov’s homoplasty of the corneal-and-conjunctival flap, Puchkovakaia’s peripheral corneal grafting; corneal-scleral-conjunctival flap plasty; labial mucosa grafting; dura matter grafting; and amniotic membrane grafting. Each of these techniques has its advantages and disadvantages [1, 14-16]. Abrupt thinning and even perforation at the limbus and cornea due to deep pterygium excision are potential postoperative complications of pterygium syrgery. This results in the development of local limbal deficiency, ulceration, perifocal corneal edema at the site of surgery, reduced vision and subsequent corneal opacity [17]. The purpose of the study was to analyze mistakes made during surgical treatment for pterygium based on medical records of patients treated at the Corneal Pathology Department of the Filatov Institute. Materials and Methods One hundred and three patients (105 eyes) who had been followed up after surgery for ptergygium stage 3 or 4 during the recent two years were involved in this study. Postoperative complications were noted in 27 eyes (25.7%). Results Of the surgical complications, there were frequent recurrences with multiple early repeat surgeries (4 to 5 surgeries within a year) with the development of extensive vascular fibrous tissue and decreased vision (10 eyes), corneal ulceration and perforation in bilateral pterygium surgery in patients with concomitant autoimmune disorders (4 cases), and corneal ulceration and perforation due to deep pterygium excision (5 cases). Example cases Case 1 A 54-year-old woman presented to the department a week after primary pterygium excision. She was diagnosed with a limbal corneal ulcer and descemetocele (Fig. 1) and underwent peripheral corneoscleral keratoplasty for tectonic reasons (Fig. 2). Nine months after keratoplasty, peripheral corneal neovascularization without fibrous ingrowth was observed (Fig. 3).
Case 2 In May, 2017, an 81-year-old lady presented to the department with a perforated corneal ulcer after primary pterygium excision. She exhibited a shallow anterior chamber, corneal edema, and uncorrected visual acuity as low as 0.05 (Fig. 4). The patient received a 4.0-mm tectonic corneoscleral keratobioimplant. At day 17 after surgery, the normal anterior chamber depth was restored, there was no corneal edema, and uncorrected visual acuity improved to 0.35 (Fig. 5). At month 5 after surgery, adequate engraftment was observed. In addition, there were no signs of pterygium recurrence, and uncorrected visual acuity improved to 0.7 (Fig. 6).
Patients with patients with a concomitant autoimmune disorder (e.g., rheumatoid arthritis) require special attention in pterygium surgery. Surgical corneal trauma may lead to formation of autoimmune complexes, resulting in collagenase activation, autoimmune keratomalacia, and even corneal perforation. Commonly, a patient exhibits no apparent signs of inflammation (irritation, pain, etc) in the course of autoimmune ulceration, and perforation is found too late, when iris prolapse occurs and the anterior chamber becomes shallow. This makes rehabilitation of these patients very difficult, especially in cases of bilateral pterygium surgery simultaneous with emergence of corneal perforation. Case 3 A 55-year-old woman presented to the department with complaints of history of vision reduction in her eye for two weeks, mucosal discharge, and dull pain. Biomicroscopy revealed hyperemia and cicatricial changes of the conjunctiva, bilateral peripheral corneal ulcers with strangulated iris, and uneven anterior chamber with deformed pupil at the site of ulcer defect (Figs. 7, 8).
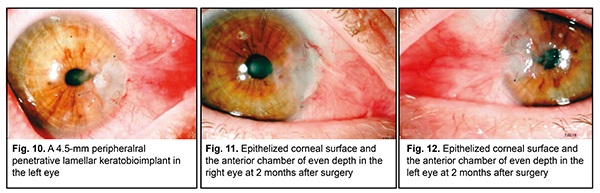
During history taking, the patient reported that she underwent an outpatient bilateral pterygium surgery without preliminary general clinical examination at her place of residence. Three days after primary pterygium surgery, she started feeling pain in her eyes. However, she sought for medical care only two weeks after surgery, in accordance with recommendations at discharge from the hospital. Surgical sutures were removed 2 weeks after surgery. A month after surgery, peripheral ulcers developed in both eyes, and the patient was sent to the institute for treatment. Detailed history analysis revealed that she had a five-year history of rheumatoid arthritis with irregular inpatient treatment courses at medical facilities specializing in care for this disease. She was taking chronic methotrexate. On admission, the following test results were presented: C-reactive protein (CRP), 9 mg/l; rheumatoid factor, 60 IU/ml; ASO, 200 IU/ml. Uncorrected visual acuity (UCVA) OD, 0.3; Best-corrected visual acuity (BCVA) OD, 0.35 with 2.0 D correction; UCVA OS, 0.2; BCVA OS, 0.4 with 2.0 D correction. IOP, hypotony on palpation OU; Schirmer test OD, 4.0 mm; Schirmer test OS, 5.0 mm; Norn test OD, 5 sec; Norn test OS, 5 sec; sensitivity of both corneas; the patient had moderately decreased corneal sensitivity in both eyes. She underwent therapeutic penetrating and lamellar keratoplasties with 3.5-mm and 4.5-mm frozen-dried corneal transplants for OD and OS, respectively (Figs. 9, 10).
This treatment resulted in restoration of corneal integrity in both eyes. Both transplants and the corneal surface were completely epithelized. The anterior chamber was of moderate and even depth. The pupil was round and mobile. UCVA OD: 0.35; UCVA OS: 0.3. The IOP normalized (Figs. 11, 12).
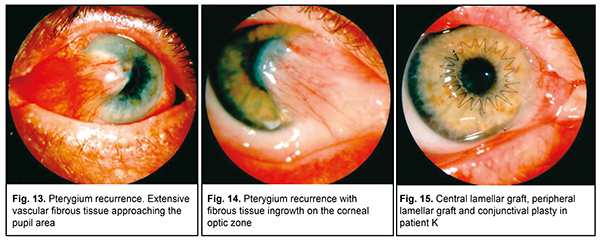
Some patients seeking care at our institute had undergone 4-5 previous surgeries for pterygium in one eye. This resulted in the development of extensive vascular fibrous tissue approaching or going beyond the pupil area, thus leading to abrupt loss of visual acuity due to marked astigmatism or obscuration of the optic zone by the pterygium head (Fig. 13). Unfortunately, chemotherapeutic methods of pterygium recurrence prevention were rarely used in surgery for recurrent pterygium. Peripheral layer-by-layer barrier keratoplasty and amniotic membrane transplantation are used in extensive and progressive pterygium or repeat recurrences of pterygium [18]. In some cases of pterygium obscuring the optic zone, peripheral lamellar keratoplasty combined with optic lamellar keratoplasty was performed after pterygium excision (Figs. 14-18). Therefore, the analysis of mistakes made in the surgical treatment of pterygium enabled recommendations to be made regarding the following: the patient should be referred to the medical facility at which he/she can undergo keratoplasty if complications (perforations and/or ulcers) develop during pterygium excision.
Conclusion First, pterygium should be excised with minimal trauma to the limbus and cornea. Second, a patient’s concomitant disease should be taken into account. Special attention should be given to autoimmune disorders, in which a corneal trauma may result in keratomalacia. Third, one should not perform bilateral pterygium surgery or repeat pterygium excision early (? 6 months) after primary pterygium surgery. Fourth, current medicamentous and surgical techniques (steroid and antimetabolic therapy), layer-by-layer barrier keratoplasty, amniotic membrane transplantation, and optic keratoplasty combined with peripheral keratoplasty should be used to prevent pterygium recurrence. Finally, any patient after pterygium excision should be followed by the ophthalmologist to promptly prevent or arrest the recurrence of pterygium. References 1.Isyaku M. Treatment of pterygium. Annals African Medicine. 2011;10(3):197-203. 2.Aliyev A-G. [Features of corneal aberrations in pterygium]. [Cand Sc (Med) Thesis]. Moscow: Research Institute for Eye Diseases; 2008. 117 p. Russian. 3.Bilalov EN. [Changes in biochemical characteristics of the tear fluid as a factor of the pathogenesis of pterygium]. Klinicheskaiia oftalmologiia. 2005;6(3):123-5. Russian. 4.Bilalov EN, Bakhritdinova FA. [Local microcirculation in persons with primary pterygium, as evidenced by fluorescein-angiographic studies]. Vestn Oftalmol. 2005 Nov-Dec;121(6):14-7. Russian. 5.Makeeva GA. [Tear production and pterygium]. Zdravookhraneniie Kazakhstana. 1981;6(423):27-9. Russian. 6.Bakbardina LM, Bakbardina II. [Peripheral barrier keratoplasty with biologically protected tissue bed in treatment of recurrent pterygium]. Oftalmol Zh. 2004;5:83-5. Russian. 7.Veselovs’ka NM, Lovtsova OD. [Method of microsurgical treating for pterygium]. Visnyk Vinnytskogo NMU. 2006;10(2):379. Ukrainian. 8.Erlyshev PA. [Operative treatment of pterygium with mobilization of the healthy conjunctiva]. Vestn Oftalmol. 1972;6:76-7. Russian. 9.Usov Via, Maltsev EV, Kritsun NIu. [Clinical efficacy of pterygium surgery technique involving use of high frequency welding of biological tissues for fixing free limbal conjunctival autograft]. Oftalmol Zh. 2015;6:6-12. Ukrainian. 10.Wit DD, Athanasiadis I, Sharma A, Moore J. Sutureless and glue-free conjunctival autograft in pterygium surgery: a case series. Eye. 2010;24:1474–7. 11.Borodin IuI, Val’skii VV, Verigo EN. [Late outcomes of combination treatment of recurrent pterygium]. Oftalmologiia. 2007;3(4):29-33. Russian. 12.Korets K. [Treatment of recurrent pterygium]. Vestn Oftalmol. 1970 Jan-Feb;1:84-5. Russian. 13.Bandyopadhyay R, Nag D, Mondal SK, Gangopadhyay S, Bagchi K, Bhaduri G. Ocular surface disorder in pterygium: Role of conjunctival impression cytology. Indian J Pathol Microbiol. 2010 Oct-Dec;53(4):692-5. 14.Fallah MR, Golabdar MR, Amozadeh J, et al. Transplantation of conjunctival limbal autograft and amniotic membrane vs. mitomycin C and amniotic membrane in treatment of recurrent pterygium. Eye. 2008 Mar;22(3):420-4. 15.Ye J, Kook KH, Yao K. Temporary amniotic membrane patch for the treatment of primary pterygium: mechanism of reducing the recurrence rate. Graefe’s Arch Clin Exp Ophthalmology. 2006 May;244(5):583-8. 16.Dzunic B, Jovanovich P, Vaselinovic D. Analysis of pathophysiological characteristics of pterygium. Bosnian J Basic Medical Sciences. 2010;10(4):307-13. 17.Buchko OIa, Tsyganova TA, Shishkin MM. [Analysis of early and late postoperative complications after pterygium excision by the technique of McReynolds]. In: Proceedings of the conference marking the 110th anniversary of the Moscow Helmholtz Research Institute for Eye Diseases. 7-8 October, 2010. Moscow, p.35-8. Russian. 18.Belyi IuA, Tereshchenko AV, et al. [A method for treating pterygium]. Novoiie v oftalmologii. 2008;(2):59. Russian. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|