J.ophthalmol.(Ukraine).2019;4:64-68.
|
http://doi.org/10.31288/oftalmolzh201946468 Peroxidation processes in ocular surface tissues in experimental hypothyroidism Drozhzhyna G.I.1, Dr. Sc. (Med.), Prof.; Pavlovskii M.I 2, a Postgraduate Student; Pavlovskaia G.Ia.3, Cand. Sc. (Med.) 1 SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) 2 Lviv regional clinical hospital;Lviv (Ukraine) 3 Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) E-mail: lvivmic87@gmail.com TO CITE THIS ARTICLE: Drozhzhyna GI, Pavlovskii MI, Pavlovskaia GIa. Peroxidation processes in ocular surface tissues in experimental hypothyroidism. J.ophthalmol.(Ukraine).2019;4:64-68.http://doi.org/10.31288/oftalmolzh201946468
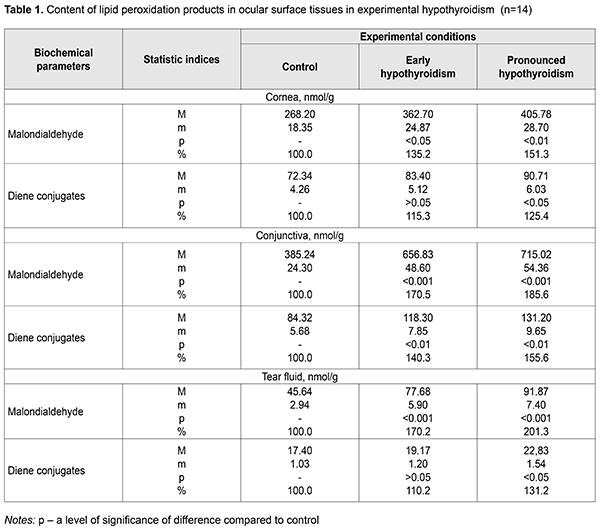
Background. It is known that a decreased level of thyroid hormones (TH) in the body causes hypothyroidism and is accompanied by metabolic, functional and structural changes in different organs and tissues, including an eye and tear glands, in particular. However, these changes in the conjunctiva, cornea, and tear fluid have not been studied. It has been shown in experiment that the decreased TH level can lead to the development of dry eye syndrome. The thyroid hormones induce the changes in the tear fluid and have an effect on the epithelium of ocular surface tissues although the mechanisms of this effect are understudied. The changes in the ocular surface tissues in the presence of hypothyroidism are also unclear. Purpose. To study experimentally the state of lipid peroxidation processes in the cornea, conjunctiva, and tear fluid on a model of hypothyroidism in rats. Material and Methods. Male white Wistar rats were used in the experiment. Forty-two animals were randomly divided into 3 groups: group 1, fourteen rats serving as control; group 2, fourteen rats with early hypothyroidism; group 3, fourteen rats with pronounced hypothyroidism. Hypothyroidism was induced by antithyroid tiamazole which the rats in the study groups received with drinking water (500 mg/l) for four and ten weeks to simulate early and pronounced hypothyroidism, respectively. We evaluated spectrophotometrically the content of malondialdehyde and diene conjugates in the tissues studied [5]. Results. In experimental hypothyroidism, the lipid peroxidation (LPO) processes are activated in the ocular tissues, which is evidenced by the increased content of malondialdehyde and diene conjugates in the conjunctiva by 85.6% and 55.6%, respectively, and in the cornea by 51.3% and 25.4%, respectively, especially in pronounced hypothyroidism. The highest rates of malondialdehyde were noted in pronounced hypothyroidism. The sharpest increase of malondialdehyde was in the tear fluid: by 70.2% and 101.3% in early and pronounced hypothyroidism, respectively. Conclusions. The increased content of malondialdehyde in the cornea, conjunctiva, and, especially, tear fluid, gives evidence of the activated LPO processes and depends on the stage of experimental hypothyroidism. The data obtained make it possible to consider a level of malondialdehyde in the tear as a biochemical indicator of damage to ocular surface tissues in hypothyroidism. Keywords: hypothyroidism, cornea, conjunctiva, tear fluid, malondialdehyde, diene conjugates, experiment
Introduction Hypothyroidism is a clinical syndrome which is caused by a persistent deficit of thyroid hormones in the body. Hypothyroidism is the most common pathology among thyroid disorders and can be diagnosed rather late due to nonspecific clinical signs at early stages. Hypothyroidism incidence in the general population is 3.7-4.6% and depends on gender, age, and iodine consumption rates. Hypothyroidism more often affects older women with a rate of 12.0-21%. Manifest hypothyroidism is noted in 0.2-2%. Subclinical hypothyroidism is defined in 7-17% and 2-3% of females and males, respectively. Primary hypothyroidism is observed in 95% of patients and among the most common causes are Hashimoto's thyroiditis, thyroid surgeries, and radioactive iodine treatment [7, 12, 16]. The incidence of hypothyroidism actualizes this problem not only for endocrinologists but for other specialists, including ophthalmologists. Thyroid hormones (TH) play an important role in the regulation of various metabolic processes in the body. Thyroid hormone deficiency leads to disorders in protein, carbohydrate, lipid, water-salt, and energy metabolism in the body. One of the main TH functions is the regulation of cellular respiration. In the presence of TH deficiency, oxygen consumption in tissues, as well as energy utilization, is decreased, which leads to the development of oxidative stress [14]. It is known that hypothyroidism is caused by a decreased level of thyroid hormones in the body which is accompanied by metabolic, functional and structural changes in different organs and tissues, including an eye and tear glands, in particular. Experimental studies have demonstrated that a decreased TH level causes dry eye syndrome (DES). THs induce changes in the tear gland and have a direct effect on the epithelium of the ocular surface tissues. However, the mechanisms of this effect are not understood. The changes in the ocular surface tissues in the presence of hypothyroidism are also unclear [1, 3, 9, 11, 17]. Analysis of literature sources and our observations give the evidence that different ophthalmic alterations are common clinical manifestations of hypothyroidism and indicative of its severity. Ophthalmic examinations are crucial for diagnosing hypothyroidism as well as for assessing its severity and treatment efficacy in patients with hypothyroidism [16, 19]. It should be noted that studying the processes of peroxidation in the anterior eye tissues in the conditions of hypothyroidism development can contribute to the search for new effective correction for metabolic and functional disorders in hypothyroidism. Purpose of the present paper was to study experimentally the state of lipid peroxidation processes in the cornea, conjunctiva, and tear fluid on a model of hypothyroidism in rats. Material and Methods Male white Wistar rats, weighed 193-210 g, were used in the experiment. The work followed International Guiding Principles for Biomedical Research Involving Animals which were provided by the Council for International Organization of Medical Sciences (2012). 42 animals were randomly divided into 3 groups: group 1, fourteen rats serving as control; group 2, fourteen rats with early hypothyroidism; group 3, fourteen rats with pronounced hypothyroidism. Hypothyroidism was induced by antithyroid tiamazole which the rats in the study groups received with drinking water (500 mg/l). To simulate early hypothyroidism, the animals received tiamazole for four weeks [6, 13]. Pronounced hypothyroidism was modeled by the ten-week intake of tiamazole [8]. On hypothyroidism onset, the animals of both groups were humanely killed with excess anesthesia using ethylic ether. Corneal and conjunctival tissues were collected for studying while tear fluid was collected using the modified Schirmer test before the animals were taken out of the experiment [8]. We evaluated spectrophotometrically the content of malondialdehyde and diene conjugates in the tissues studied [5]. The findings on the content of malondialdehyde and diene conjugates in the tissues of the cornea, conjunctiva, and tear fluid were statistically processed using the SPSS 11.0 package [2]. Results Data on the content hypothyroidism of lipid peroxidation products in the ocular surface tissues in experimental hypothyroidism are demonstrated in Table 1.
The content of malondialdehyde in the cornea of the animals with early hypothyroidism was increased up to (362.70±24.87) nmol/g, which was 135.2% as compared to control, (268.20±18.35) nmol/g, (р<0.05). In pronounced hypothyroidism, the content of malondialdehyde increased up to (405.78±28.70) nmol/g, equalling 151.3% comparing to control (р<0.01). The content of diene conjugates in the cornea of the animals with early hypothyroidism was increased up to (83.40±5.12) nmol/g, which was 115.3% as compared to control, (83.40±5.12) nmol/g. In pronounced hypothyroidism, the content of diene conjugates increased up to (83.40±5.12) nmol/g, equalling 125.4% comparing to control (р<0.05). As shown in Table 1, the content malondialdehyde in the conjunctiva of the animals with early hypothyroidism was increased up to (656.83±48.60) nmol/g, which was 170.5% as compared to control, (385.24±24.30) nmol/g, (р<0.001). In pronounced hypothyroidism, the content of malondialdehyde increased up to (715.02±54.36) nmol/g, equalling 185.6% hypothyroidism was increased up to (118.30±7.85) nmol/g, which compared to control (р<0.001). The content of diene conjugates in the conjunctiva of the animals with early hypothyroidism was 140.3% as compared to control, (84.32±5.68) nmol/g. In pronounced hypothyroidism, the content of diene conjugates increased up to (131.20±9.65) nmol/g, equalling 155.6% comparing to control (р<0.01). In the tear fluid of the experimental animals, the content malondialdehyde was increased up to (77.68±5.90) nmol/g in early hypothyroidism, which was 170.2% as compared to control, (45.64±2.94) nmol/g, (р<0.001). In pronounced hypothyroidism, the content of malondialdehyde increased up to (91.87±7.40) nmol/g, equalling 201.3% comparing to control (р<0.001). The content of diene conjugates in the tear fluid of the animals with early hypothyroidism was increased up to (19.17±1.20) nmol/g, which was 110.2% as compared to control, (17.40±1.03) nmol/g. In pronounced hypothyroidism, the content of diene conjugates increased up to (22.83±1.54) nmol/g, equalling 131.2% comparing to control (р<0.05). Discussion Summarizing the data on end and intermediate products of lipid peroxidation, malondialdehyde and diene conjugates, respectively, it can be concluded that hypothyroidism activates significantly the processes of peroxidation in ocular tissues. This leads to an accumulation of lipid hydroperoxides in the tear fluid, conjunctiva, and cornea. A rate of how the level of lipid peroxidation products, and mainly of end-product malondialdehyde, is increased depends on a stage of hypothyroidism. The highest malondialdehyde rates are characteristic for pronounced hypothyroidism. Specific mention should be made of a sharp rise in the concentration of malondialdehyde in the tear fluid in all stages of experimental hypothyroidism. In prospect, this fact can be used as an element of diagnosis for ocular surface damage in the underactive thyroid gland. It is interesting to compare our findings with literature data on a state of lipid peroxidation processes and antioxidant system in tear gland tissues [8]. The mentioned paper on studying lipid peroxidation processes, a glutathione level, and peroxidases activity in the tear fluid in experimental hypothyroidism has demonstrated that underactive thyroid gland leads to an increased level of glutathione and a high concentration of malondialdehyde in the studied tissues. In addition, peroxidase activity was three-fold decreased. All these facts are an important element in misbalance between the reduction system of glutathione and processes of oxidative injury of lipids since glutathione is known to decrease the intensity of lipid peroxidation and the accumulation of lipid peroxidation products through a glutathione peroxidase reaction, in which it reduces lipid hydroperoxides. Herewith, glutathione transforms into an oxidized form [4, 8, 18]. Thus, our findings in conjunction with literature data give the evidence that in hypothyroidism there is dysregulation in the processes of lipid peroxidation and the antioxidant system that results in oxidative stress which is known to have a negative effect on the function and protective and adaptive mechanisms of ocular tissues [8, 10, 11, 15, 20] and can be one of pathogenetic elements in the development of dry eye syndrome in hypothyroidism. In conclusion, firstly, it was found that the rats with experimental hypothyroidism had the activated lipid peroxidation processes in the ocular tissues, which is evidenced by the increased content of malondialdehyde and diene conjugates in the conjunctiva by 85.6% and 55.6%, respectively, and in the cornea by 51.3% and 25.4%, respectively, especially in pronounced hypothyroidism. Secondly, it was found that in experimental hypothyroidism the highest rates of malondialdehyde and diene conjugates were noted in the tear fluid: by 70.2% and 101.3% in early and pronounced hypothyroidism, respectively. This parameter can be considered as a diagnostic criterion characterizing a state of oxidative stress in ocular surface tissues in hypothyroidism even in the early stage of hypothyroidism development. References 1.Horodynska OYu, Bobyriova LYe. [Prognostic characteristics of hypothyroidism prevalence in the poltava region and in ukraine under conditions of iodine deficiency]. Mizhnar. endokryn. zhurn. 2016;2(74):44-8. In Ukrainian. 2.Nasledov A. [SPSS computer data analysis in psychology and social science]. Spb.:Piter;2005. 416p. In Russian. 3.Pavlovska GYa, Pavliv OB, Pavlovskii MI, Paster SYa. [Dry keratoconjunctivitis in patients with hypothyroidism]. [Proceedings of XIII Ukrainian Congress of Ophthalmologists. 21-23 May 2014]. Odessa; 2014:37-8. In Ukrainian. 4.Babu K, Jayaraaj IA, Prabhaka J: Effect of Abnormal thyroid hormone changes in lipid peroxidation and Antioxidant imbalance in Hypothyroid and Hyperthyroid patients. Int J Biol Med Res. 2011; 2(4): 1122-6. 5.Bergmeyer HU. Methoden der enzymatischen Analyse. Berlin; 1986. 2220 p. 6.Cano-Europa E, Blas-Valdivia V, Lopez-Galindo GE. Methimazole-induced hypothyroidism causes alteration of the REDOX environment, oxidative stress, and hepatic damage; events not caused by hypothyroidism itself. Ann Hepatol. 2010 ;9(1): 80-8. 7.Cooper DS. Antithyroid drugs. N Engl J Med. 2005; 352(9): 905-17. 8.Dias AC, M?dulo CM, Jorge AG, Braz AM. Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest Ophthalmol Vis Sci. 2012; 52(7): 3038-42. 9.Gatzioufas Z, Panos GD, Brugnolli E, Hafezi F. Corneal topographical and biomechanical variations associated with hypothyroidism. J Refract Surg. 2014;30(2):78-9 10.Kumari SN, Sandhya, Damodara Gowda KM. Oxidative Stress in Hypo and Hyperthyroidism. Al Ame en J Med Sci. 2011; 4(1): 49-53. 11.Micali A, Pisani A, Puzzolo D, Spinella R. Effect of hypothyroidism on postnatal conjunctival development in rats. Ophthalmic Res. 2011; 45(2): 102-12. 12.Mohanty S, Amruthlal W, Reddy G. C. Diagnostic strategies for subclinical hypothyroidism. Indian J Clin Biochem. 2010; 25(3): 279-82. 13.Ortiz-Butron R, Pacheco-Rosado J, Hern?ndez-Garcia A. Mild thyroid hormones deficiency modifies benzodiazepine and mu-opioid receptor binding in rats. Neuropharmacology. 2013; 54(1): 111-6. 14.Ozturk BT, Kerimoglu H, Dikbas O. Ocular changes in primary hyperthyroidism. BMC Reseach Notes. 2010; 3: 266-71. 15.Petruela M, Muresan A, Duncea I. Oxidative Stress and Antioxidant Status in Hypo- and Hyperthyroidism. Antioxidant Enzymes. Licensee InTech. 2012; 8: 197-236. 16.Plummer CE, Specht A, Gelatt KN. Ocular manifestations of endocrine disease. Compend Contin Educ Vet. 2013; 31(12): 733-43. 17.Shashikala P. Prevalence of dry eye in hypothyroidism. Int J Clin Cases Investigations, 2013; 5 (1): 46-51. 18.Venditti P, Balestrieri M, Di Meo S, De Leo T. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J Endocrinol. 1997; 155(1): 151-7. 19.Villanueva I, Alva-S?nchez C, Pacheco-Rosado J. The Role of Thyroid Hormones as Inductors of Oxidative Stress and Neurodegeneration. Oxidative Medicine and Cellular Longevity. 2013; 13: 1-15. 20.Yilmaz S, Ozan S, Benzer F, Canatan H. Oxidative damage and antioxidant enzyme activities in experimental hypothyroidism. Cell Biochem Funct. 2003; 21(4): 325-30.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|