J.ophthalmol.(Ukraine).2019;4:57-63.
|
http://doi.org/10.31288/oftalmolzh201945763 The effect of high intraocular pressure on enzymatic antioxidant system of uveal tissues in rabbits with experimental allergic uveitis I.M. Mikheitseva, Dr. Sc. (Biol.); N.V. Bondarenko, a Postgraduate Student; S.G. Kolmiichuk, a Research Fellow; T.I. Siroshtanenko, a Junior Research Fellow; M.M. Trofimov, Cand. Sc. (Vet.); L.V. Zagrodska, a Laboratorian SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mikheitseva IM, Bondarenko NV, Kolmiichuk SG, Siroshtanenko TI, Trofimov MM, Zagrodska LV. The effect of high intraocular pressure on enzymatic antioxidant system of uveal tissues in the rabbits with experimental allergic uveitis. J.ophthalmol.(Ukraine).2019;4:57-63. http://doi.org/10.31288/oftalmolzh201945763
Background. Determining the influential mechanisms of high intraocular pressure on the development and course of non-infectious anterior uveitis is a relevant but understudied issue which requires a detailed study. Of particular interest, in this regard, are free radical mechanisms which can be a trigger for oxidative stress and cause damage to cell membranes in ocular tissues. Purpose. To study the activity of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and catalase) in uveal tissues and chamber aqueous humor in rabbits with experimental non-infectious anterior uveitis with and without ocular hypertension. Material and Methods. The experiment involved forty-one animals divided into 4 study groups: Group 1 (n = 10), ocular hypertension (OH); Group 2 (n = 10), uveitis (U); Group 3 (n = 12), (OH+U); Group 4 (n = 9), intact animals as control. Allergic uveitis was simulated using our method through the administration of bull serum albumin with a dose of 5 mg in previously sensibilized animals. To simulate ocular hypertension in the experimental groups 1 and 3, the animals were made a single 0.1 ml injection of 0.3% carbomer in the anterior chamber. Biochemical studies were conducted spectrophotometrically for the tissues of the uvea (the iris and the ciliary body) and the aqueous humour. The activity of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and catalase) was determined. Parametric statistical tests with Student's t-test using the Statistica package were used to process the data. Results. Ocular hypertension in experimental non-infectious anterior uveitis resulted in a more intensive decrease in the activity of the enzymatic antioxidant system (by 19.4 and 20.9% for superoxide dismutase; by 25.5 and 21.9% for glutathione peroxidase; and by 14.3 and 19.6% for catalase) in the aqueous humor and uveal tissues, respectively, compared to the rabbits with uveitis and normal intraocular pressure. Conclusions. A decrease revealed in the activity of superoxide dismutase, catalase, and, especially, glutathione peroxidase testifies to depletion of the enzymatic antioxidant system in experimental conditions of increased peroxidation in the uveal tissues in ocular hypertension. Our findings confirm the assumption that elevated intraocular pressure can be a factor contributing to severe inflammation in the anterior eye. One of the major cell mechanisms inducing such pathological conditions is oxidative stress. Keywords: ocular hypertension, uveitis, glutathione peroxidase, superoxide dismutase, catalase, rabbits
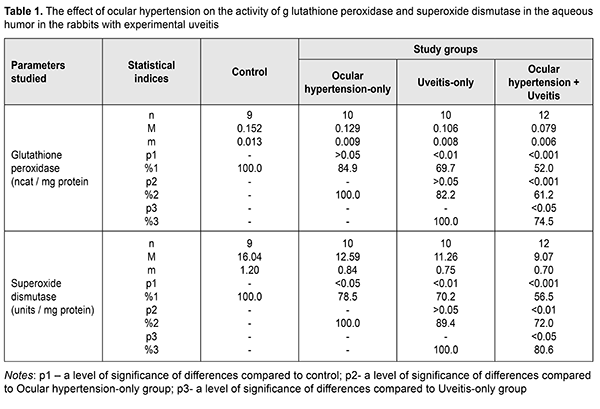
Background Uveitis is one of the most severe eye conditions. Secondary glaucoma, being one of the major uveitis complications, develops in 10-60% and leads to visual loss in people often of the working age. An increase of intraocular pressure (IOP) in patients with chronic uveitis causes the development of uveal ocular hypertension and post-uveal glaucoma [1, 2, 3]. According to the literature, secondary glaucoma is a common complication in patients with anterior uveitis. Increased IOP is noted in patients with chronic recurrent uveitis, Fuchs' dystrophy, and herpes simplex virus-associated uveitis [4]. Elevated IOP develops in approximately 12.0% at 3 months after acute uveitis and in 27.0% in chronic uveitis [4, 5, 6]. A short-term IOP increase is not always an indicator of glaucoma so it is of great importance to reveal risk factors for secondary glaucoma in such patients. Glaucoma cannot be revealed in most cases of uveitis despite the alterations revealed in the anterior chamber angle [1]. Elevated IOP can be caused by many factors since uveitis itself and uveitis treatments with corticosteroids can affect IOP. In uveitis, a short-term IOP increase is more often associated with inflammation in the trabecular meshwork. Inflammation-related changes are a narrowing of trabecular spaces between trabecular fibers and endothelial cells due to swelling. In the development of uveal ocular hypertension, of a certain value are an increase in the permeability of blood vessels and an increase in the amount of fluid, containing a great number of proteins, in the eye [1, 5, 7]. Today, major components of primary open-angle glaucoma pathogenesis are considered to be i) elevated IOP which can be associated with local functional and dystrophic changes in aqueous outflow; ii) damage to hydrostatics and hydrodynamics of the eye; iii) secondary vascular and dystrophic changes in ocular tissues and the optic nerve; iv) optic nerve atrophy [8, 9, 10, 11]. According to the literature, disorders in the blood supply in the eye is an important cause of progressive vision loss in patients with elevated IOP. Several studies on this subject have demonstrated that dynamic pressure in the ophthalmic artery is maintained at the required level due to contraction of the muscle layer in the vessel walls and an increase of vascular tone. Due to severe sclerotic alterations in the ophthalmic artery walls and intraocular vessels (small arteries and arterioles), there is a vascular dysregulation which affects ocular blood flow and contributes to the pathological process [6, 9, 11]. Recently, the greatest attention in the pathogenesis of hypertension and glaucoma is paid to primary or secondary metabolic disorders [3, 7, 12]. The most important component of glaucoma pathogenesis is an exogenous and endogenous imbalance in free-radical oxidation of biomolecules and potential of the antiradical system [3, 7, 11, 12, 13]. Primary metabolic changes independent on an IOP level conform to metabolism and age-related and concomitant conditions of an individual patient. Secondary metabolic changes develop in a result of a direct action of elevated IOP on intraocular structures and cause hypoxia, ischemia, dystrophic changes in the drainage system, the retina, and the optic nerve [8, 9, 10, 11]. Single publications are elucidating a possible role of inflammation as a causal factor in the pathogenesis of glaucoma [14]. Thus, autoimmune aggression was revealed in various forms of glaucoma. Normal-tension glaucoma affects retinal photoreceptors while in primary open-angle glaucoma ganglion cells are affected. It is quite possible that a difference revealed autoimmune aggression in various forms of glaucoma is associated with the character of hemodynamic disorders which induce ischemia of retinal layers [15]. Slepova O.S. and colleagues (2016) have defined disorders of the cytokine status at both local and systemic levels while comparing the age-related changes with the absence of ophthalmic pathology and in patients suspected primary open-angle glaucoma. The data obtained testify to the fact that disorders in cytokine regulation at early stages of primary open-angle glaucoma occur even before clinical manifestations of the disease [3, 15]. At the same time, determining the initial impact of elevated IOP on intraocular inflammation, the formation of uveitis signs, and uveitis course is an understudied issue. In our opinion, it is important to determine not only the presence of this impact but to study its pathogenic mechanisms. Previously, we have found experimentally that in a model of allergic uveitis against the background of high IOP there are much more activated lipid peroxidation (LPO) processes with toxic LPO products accumulated in the anterior segment of the eye. The purpose of the present paper was to study the effect of elevated IOP (ocular hypertension) on the activity of antioxidant enzymes in uveal tissues and chamber aqueous humor in experimental non-infectious anterior allergic uveitis. Material and Methods The experiment was conducted on Chinchilla rabbits, weighed 3-3.5 kg. The animals were kept under standard vivarium conditions and received food and water ad libitum. The experiment followed the General Ethical Principles of Animal Experiments (Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986). The experiment involved forty-one animals divided into 4 study groups. Group 1 included 10 animals with experimental ocular hypertension; group 2 included 10 animals with experimental allergic uveitis; group 3 included 12 animals with ocular hypertension and allergic uveitis; and group 4 included 9 intact animals serving as controls. To simulate ocular hypertension in the experimental groups 1 and 3, the animals were made a single 0.1 ml injection of 0.3% carbomer in the anterior chamber [16]. To simulate allergic uveitis, the animals underwent general anesthesia through 50 mg/kg ketamine administration, following local eye drop instillations of 0.5% procaine hydrochloride, instilled into the conjunctival sac 1minute earlier. Allergic uveitis was modeled using our method through the administration of bull serum albumin with a dose of 5 mg in previously sensibilized animals [17]. IOP in the rabbits was measured using a contact Maklakov tonometer with a 7.5 g plunger under local anesthesia (0.5% proxymetacaine hydrochloride). IOP levels were (13.5±0.6) mmHg in controls; (18.7±1.2) mmHg in the ocular hypertension-only group; (15.3±1.4) mmHg in the anterior uveitis-only group; and (23.4±1.3) mmHg in the ocular hypertension and anterior uveitis group. The animals were taken out of the experiment under deep anesthesia (10% thiopental sodium, 1ml/1kg) by air embolism. The eyes were enucleated at the temperature of 0?С-5?С. Biological material for biochemical studies was collected at 4 weeks on completion of ocular hypertension and anterior uveitis simulation. Biochemical studied were conducted for the tissues of the uvea, i.e. the iris and the ciliary body used to prepare homogenate using 0.9% sodium chloride with the ratio of 1:9 (weight: volume), and the aqueous humour. The obtained extracts were centrifuged with 1000 r.p.m at 5 ?С for 10 minutes. The determination of superoxide dismutase is based on determining the rate the enzyme inhibits by the reduction of nitrosine tetrazolium by superoxide radicals. The variation coefficient of the method is 6.2% [18]. The determination of catalase is based on the ability of hydrogen peroxide to form a stable colored complex with molybdenum salts. The variation coefficient of the method is 8.7% [19]. The activity of glutathione peroxidase was determined spectrophotometrically according to the rate of oxidized glutathione formation through conjugated reactions with the NADPN-dependent enzyme, glutathione reductase, by recording the change of optical density during NADPN oxidation at the wavelength of 340 nm. The variation coefficient of the method is 1.8% [20]. Parametric statistical tests with Student's t-test using the Statistica package were used to process the findings of the experimental studies [21]. Results and Discussion The activity of antioxidant enzymes, glutathione peroxidase, superoxide dismutase and catalase, were studied in the anterior segment of the eye in the experimental rabbits with allergic uveitis-only, ocular hypertension-only, and uveitis against ocular hypertension. The activity of the enzymatic antioxidant system in the uveal and aqueous humor tissues was decreased in the three experimental groups as compared to the norm; however, the changes were different for different groups. The data on the activity of glutathione peroxidase and superoxide dismutase in the aqueous humor of the experimental rabbits are given in Table 1. The glutathione peroxidase activity was increased in the ocular hypertension-only and uveitis-only groups by 15.1% (р>0.05) and 30.3% (р<0.01), respectively, as compared with the intact animals serving as control. The glutathione peroxidase activity in the aqueous humor of the animals with ocular hypertension and uveitis was found to be even more decreased: by 48.0% (р<0.001) as compared to control and by 38.8% (р<0.001) and 25.5% (р<0.05) as compared to ocular hypertension-only and uveitis-only, respectively.
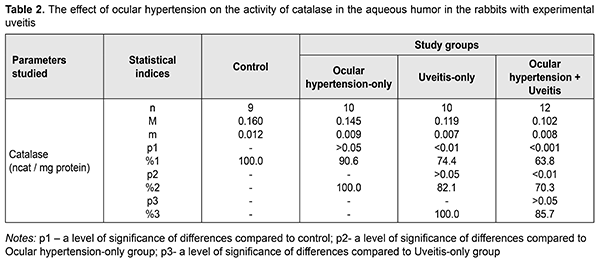
The superoxide dismutase activity in the aqueous humor in the rabbits with ocular hypertension-only and uveitis-only decreased, compared with control, by 21.5% (р<0.05) and 29.8% (р<0.01), respectively. In the ocular hypertension and uveitis rabbits, the superoxide dismutase activity in the aqueous humor decreased significantly: by 43.5% (р<0.001) compared with control and by 28% (р<0.01) and 19.4% (р<0.05) compared with the rates with ocular hypertension-only and uveitis-only, respectively. The data on the activity of catalase in the aqueous humor of the experimental rabbits with ocular hypertension and uveitis are given in Table 2. The catalase activity in the aqueous humor tended to decrease, however, not significantly, in ocular hypertension-only and decreased significantly, by 25.6% (р<0.01), in uveitis-only as compared with control. The decreased catalase activity was noted in the experimental rabbits with both ocular hypertension and uveitis as compared to control, ocular hypertension-only, and uveitis-only: by 36.2% (р<0.001), 29.7% (р<0.001), and14.3% (р>0.05), respectively.
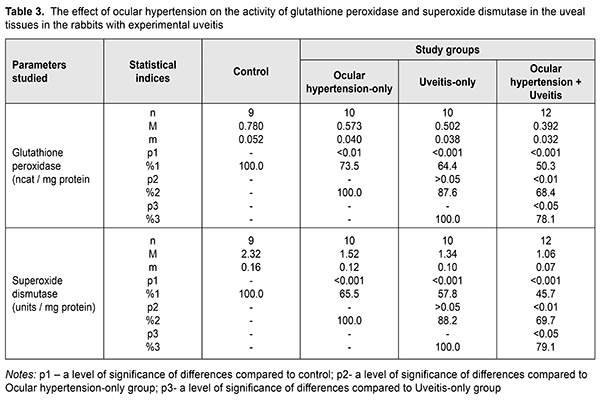
Tables 3 and 4 present the data on the effect of ocular hypertension on the activity of glutathione peroxidase, superoxide dismutase and catalase in the uveal tissues in allergic uveitis, revealing more pronounced changes in the antioxidant enzyme activity compared to those in the aqueous tissues. The glutathione peroxidase activity was decreased in ocular hypertension-only, uveitis-only, and ocular hypertension+uveitis, compared with control, by 26.5% (р<0.01), 35.6% (р<0.001), and 49.7% (р<0.001), respectively.
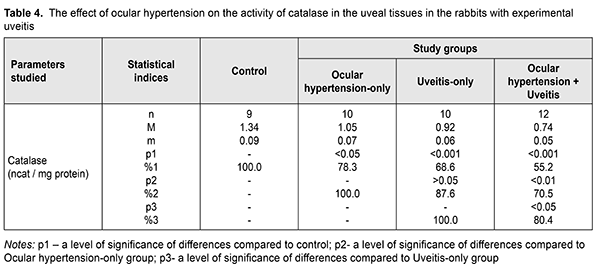
Comparing the glutathione peroxidase activity in the ocular hypertension+uveitis rabbits with that in the ocular hypertension-only and uveitis-only rabbits revealed a statistically significant decrease by 31.6 (р<0.01) and 21.9% (р<0.05), respectively. Table 3 demonstrates the superoxide dismutase activity in the uveal tissues which was significantly decreased, compared with control, in the ocular hypertension-only, uveitis-only, and ocular hypertension+uveitis rabbits by 34.5% (р<0.001), 42.2% (р<0.001), and 54.3% (р<0.001), respectively. The superoxide dismutase activity in the experimental rabbits with both ocular hypertension and uveitis was lower by 30.3% (р<0.01and 20.9% (р<0.05) as compared, respectively, with the ocular hypertension-only and uveitis-only rabbits. The data on the catalase activity in the uveal tissues in the experimental rabbits with ocular hypertension and uveitis are given in Table 4. The catalase activity in the uveal tissues decreased in ocular hypertension-only and uveitis-only compared with control: by 21.7% (р<0.05) and 31.4% (р<0.001), respectively. In the ocular hypertension+uveitis rabbits, the catalase activity was significantly lower: by 44.8% (р<0.001) comparing with control and by 29.5% (р<0.01) and 19.6% (р<0.05) comparing with ocular hypertension-only and uveitis-only, respectively. Evaluating the activity of the antioxidant enzymes in the uveal and aqueous humor tissues showed the most pronounced changes, especially in the uveal tissues, were in experimental ocular hypertension against the background of uveitis. At the same time, the changes in the catalase activity were less pronounced compared with other enzymes and the activity of superoxide dismutase was lower than that of glutathione peroxidase in all tissues studied in all experimental groups. A significant decrease in the superoxide dismutase activity can contribute to the accumulation of chemically aggressive superoxide radicals of oxygen and indicate that the tissues of the anterior eye are under conditions of oxidative stress. In addition, a significant decrease in the glutathione peroxidase and catalase activity in the aqueous humor and uveal tissues in experimental uveitis in the presence of elevated IOP enhances the damaging effect of organic and inorganic peroxides on the structural and functional properties of enzymes and the membrane structures of eye tissues. In this regard, the existing oxidative stress can be a trigger for those processes that initiate the progression of both degenerative and inflammatory pathological changes [3, 7, 11, 12, 14]. In conclusion, elevated intraocular pressure (ocular hypertension) in the rabbits with anterior non-infectious allergic uveitis contributed to a more pronounced depletion of the enzymatic antioxidant system in the uveal and aqueous humor tissues, which evidenced in the decreased activity of the main antioxidant enzymes, glutathione peroxidase, superoxide dismutase and catalase. References 1.Ermakova NA. [General knowledge on uveitis pathogenesis]. RMZh Klin. Oftalmologiia. 2003;4(4):141-3. In Russian. 2.Novitskaia ES. [Early diagnosis of postuveal glaucoma and uveal ocular hypertension in patients with chronic uveitis]. RMZh Klinicheskaia Oftalmologiia. 2012;4:139-41. In Russian. 3.Slepova OS, Arapiev MU, Lovpache DN, Balatskaya NV, Kulikova IG. Specifics of local and systemic cytokine profile in healthy people of different ages and patients with early stage of primary open-angle glaucoma. National Journal glaucoma. 2016;15(1):3-12. 4.Sallam А, Sheth HG, Habot-Wilner Z, Lightman S. Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am. J. Ophthlmol. 2009;148(2):207-13. 5.Syrovaya AO, Leontieva FS, Novikova IV, Ivannikova SV. [Biological role of free radicals in development of pathological states]. Mezhdunar. Med. Zhurn. 2012;3:98-104. In Russian. 6.Ustinova EI. [Uveal (inflammatory and post-inflammatory) glaucoma (pathogenesis, clinical picture, classification, treatment)]. Oftalmol. Vedomosti. 2009;2(2):81-91. In Russian. 7.Beishenova GA, Chesnokova NB. [The role of free radical oxidation in uveitis pathogenesis (a literature review)]. Ros. Oftalmol. Zhurn. 2015;9(2):99-105. In Russian. 8.Ielskii VN, Mikheitseva IN. [Dysregulatory aspects of the glaucomatous process (Review of the literature and own investigations.] Zhurn. NAMN Ukrainy. 2011;17(3):235-44. In Russian. 9.Erichev VP, Tumanov VP, Panushkina LA. [Glaucoma and nuerodegererative diseases.] Glaukoma;2012;1:62-8. In Russian. 10.Zavgorodniaia NG, Pasechnikova NV. [Primary glaucoma: A new look at an old problem]. Zaporizhzhia: Orbita-YUG; 2010p. In Russian 11.Flammer J, Orgul S, Costa V. The impect of ocular blood flow in glaucoma. Prog. Retinal. Eye Res. 2002;21:359-93. 12.Kurysheva NI, Vinetskaia MI, Erichev VP, Demchuk ML, Kuryshev SI. [Contribution of free-radical reactions of chamber humor to the development of primary open-angle glaucoma]. Vestn Oftalmol. 1996 Sep-Oct;112(4):3-5. In Russian. 13.Gazirova IR. [The state of oxidation-reduction system in patients with primary open angle glaucoma]. Kazanskii Med. Zhurn. 2012;3:488-90. In Russian. 14.Rupali V, Tsai JC, Kolko М. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013;58(4):190. 15.Likhvantseva VG, Gabibov AA, Solomatina MV, Belogurov AA, Korosteleva EV, Vygodin VA. The role of immune reactions in the pathogenesis of optic neuropathy in normal tension glaucoma. National Journal glaucoma. 2014;13(2):17-28. In Russian. 16.Wang YY. Experimental study of carbomer glaucoma model in rabbits by injecting different location in anterior chamber. Ophthalmol. 2009;45:1-95. 17.Mikheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. Oxidation and peroxidation in the uvea of the rabbit eyes with experimental uveitis and ocular hypertension. J.ophthalmol.(Ukraine).2019;2:55-60. 18.Makarenko EV. [Comprehensive determination of superoxide dismutase and glutathione reductase activity in erythrocytes in patients with chronic liver disease]. Lab. Delo. 1988;11:48-50. In Russian. 19.Koroliuk MA, Ivanova LI, Mayorova IG, Tokarev VE. [A method for determining the catalase activity]. Lab. Delo. 1988;1:16-8. In Russian. 20.Model MA. [On determining the activity of glutathione peroxidase]. Vopr. Med. Khimii. 1989;4:132-3. In Russian. 21.Rebrova OYu. [Statistical analysis of medical data. The use of software package STATISTICA]. M.: Media Sfera; 2002. 312 p. In Russian.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|