J.ophthalmol.(Ukraine).2019;4:49-56.
|
http://doi.org/10.31288/oftalmolzh201944956 Lectin histochemical findings of modified glycome of corneal structural components in experimental hypothyroidism M. B. Shchur, Cand Sc (Med), Kh. I. Strus, Cand Sc (Biol), A. M. Yashchenko, Dr Sc (Med), Prof Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) E-mail: yashchenko_am@ukr.net TO CITE THIS ARTICLE: Shchur MB, Strus KhI, Yashchenko AM. Lectin histochemical findings of modified glycome of corneal structural components in experimental hypothyroidism. J.ophthalmol.(Ukraine).2019;4:49-56. http://doi.org/10.31288/oftalmolzh201944956
Background: Carbohydrate determinants of the cellular surface and cytoplasmic glycopolymers which enable their mutual recognition, and effect cell proliferation, cell size and movement regulation and apoptosis, have become a focus of recent attention of researchers. Lectins have been used as specific tools in the research of carbohydrate-containing biopolymers, the letter being represented in a living organism by glycoproteins, glycolipids, and polysaccharides. Purpose: To investigate the effect of experimental hypothyroidism on the histophysiological characteristics and on the glycome of corneal structural components. Materials and Methods: A total of 35 adult male Wistar rats (weight, 180-240 g; 25 experimental rats and 10 control rats) were used in the study. Experimental hypothyroidism was induced by the addition of merkazolil at 5 mg/kg of body weight to the meals daily for two weeks. Thyroid glands and eye globes were excised and fixed with Bouin’s fluid and 4% formalin. Preparations were stained with hematoxylin and eosin. Histological, general histochemical and lectin histochemical studies were conducted. Lectin histochemistry studies were performed with the use of a panel of six lectins (РNA, HPA, SNA, LABA, WGA, and CNFA). Results: We found morphological changes in the cornea of rats with experimental hypothyroidism. These changes included keratinization and local exfoliation of the surface layer of the anterior corneal epithelium, edema and lymphocyte infiltration at the site of the expanded venous sinus, local exfoliation of the anterior chamber endothelium, reduced expression of receptors of HPA and CNFA in the anterior epithelium and increased expression of receptors of these lectins in keratocytes and collagen fibrils of the corneal stroma. Accumulation of the latter may exert effects on epithelial adhesion to the basement membrane and change corneal clarity which is associated with keratocyte production of crystallines and keratan sulfates. Conclusion: We found morphological changes and modified glycome of corneal structural components in experimental hypothyroidism. Keywords: experimental hypothyroidism, rats, cornea, glycome, lectin histochemistry
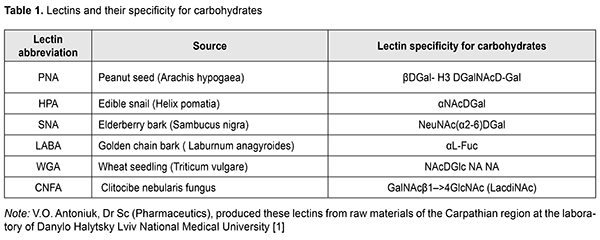
Introduction There is still no agreement among endocrinologists and ophthalmologists as to whether endocrine ophthalmopathy (EOP) is a manifestation of thyroid dysfunction or could be considered a nosological entity [1, 2]. The average annual incidence rate for EOP among females is 16.3 per 100,000, and among males, 3 per 100,000. Most authors believe that the pathogenesis of exophthalmos in EOP is influenced by three major factors: increased extraocular muscle volume due to infiltration of the endomysium by fibroblasts and lymphocytes; increased adipocyte volume in the presence of impaired adipogenesis in the orbital structures; and accumulation of glycosaminoglycans [3]. Aside of the above factors, there is an important aspect of impaired orbital venous circulation that is worth mentioning. Interrelationships between these factors cause variations in clinical symptoms, severity and activity of the process [4]. Graves’ ophthalmopathy, also called EOP, Graves' disease, autoimmune ophthalmopathy, or Graves' orbitopathy, is a an organ-specific autoimmune disease characterized by edema and lymphocytic infiltration of the retrobulbar cellular tissue and extraocular muscles, and manifested by exophthalmos of various severity as well as by ophthalmoparesis [1, 3-5]. Carbohydrate determinants of the cellular surface and cytoplasmic glycopolymers which enable their mutual recognition, and effect cell proliferation, cell size and movement regulation and apoptosis, have become a focus of recent attention of researchers [6-10]. Numerous researchers consider glycocode (a system for coding of biological information) as an important appendix to the genetic code [8, 9, 11]. Lectins have been used as specific tools in the research of carbohydrate-containing biopolymers, the letter being represented in a living organism by glycoproteins, glycolipids, and polysaccharides [6-9, 12]. Previously, the expression and distribution of glycoconjugates (receptors of lectines) in the normal rat retina has been characterized, and lectin binding to the retinal pigment epithelium (RPE) and role in the adhesion of RPE and retinal photoreceptor layer (RPL) elements between neurons and neuroglial elements have been demonstrated [13, 14]. The purpose of the study was to investigate the effect of experimental hypothyroidism on the histophysiological characteristics and on the glycome of corneal structural components. Materials and Methods A total of 35 adult male Wistar rats (weight, 180-240 g; 25 experimental rats and 10 control rats) were used in the study. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447– IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Sceintific Putposes from the European Treaty Series (Stransbourg, 1986), and approved by the Bioethics Committee of the Danylo Halytsky Lviv National Medical University (Act No. 1 dated 31.01.2018). Experimental hypothyroidism was induced by the addition of merkazolil (thiamazol; manufactured by Zdorov’ia, Kharkiv, Ukraine) at 5 mg/kg of body weight to the meals daily for two weeks. Rats were euthanized by overdosing with ether, and thyroid glands and eye globes were excised and fixed with Bouin’s fluid. Preparations were stained with hematoxylin and eosin. In order to prepare semi-thin (1-2 ?m) sections, globes were embedded in 4% formalin and dehydrated in ascending grades of alcohol and absolute acetone. Blocks were embedded in Araldite-Epon mixture. Semi-thin (1-2 ?m) sections were cut with glass knives on a UMTP-3M ultramicrotome and stained with toluidine blue. The PAS staining was used to detect neutral polysaccharides. Alcian blue staining was used to detect sulfated glycosaminoglycans at pH 2.5 [15]. Lectin histochemistry studies were performed with the use of a panel of six lectins of a broad specificity for carbohydrates (Table 1).
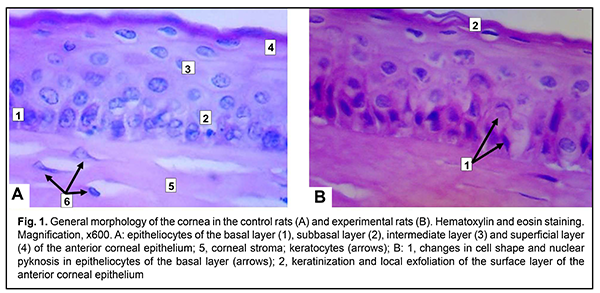
Lectin receptors were visualized in 3,3'-diaminobenzidine hydrochloride system in the presence of H2O2. Lectin peroxidase conjugates were excluded from the staining protocol to control the specificity of histochemical reactions. Morphological characteristics of thyrocytes and colloid were examined to assess thyroid gland function. Histological specimens were visualized using a Granum microscope equipped with an Echoo-Imager 502000 camera, and specimen photographs were processed with TopView 3.2 software. Microsoft Office Exсel 2003 and STATISTICA 6 software were used for statistical analyses [16]. Data are presented as mean and standard error. The levels of significance were taken at *p < 0.05, **p < 0.01, and ***p < 0.001. Results Morphological characteristics of thyrocytes and colloid were examined to assess thyroid gland function and thus to make sure that the hypothyroidal status was achieved. In the experimental animals, thyroid glands were macroscopically found to be increased two to three fold compared to the controls. In addition, microscopy found that thyroid follicles in the experimental animals, but not in controls, had an irregular shape (appeared folded), and no or small amounts of colloid. Moreover, the cubical epithelium of thyroid follicles in controls was cylinder-shaped. Thus, in the experimental animals, mean thyrocyte height was greater than in the controls (3.29±0.29 ?m vs 9.31±0.49 ?m, р < 0.001), thyrocyte hyperplasia was observed, and the thyroid was hyperemic. Examination of H&E stained corneal specimens demonstrated that the structure of the cornea in control rats was similar to that of the human cornea, and comprised the non-keratinized anterior epithelium, anterior basement membrane, proper substance (stroma), posterior basement membrane, and endothelium (a single-layer of flattened epithelial cells located on the posterior surface of the cornea) (Fig. 1A).
In addition, in the rats with merkazolil-induced hypothyroidism, keratinization and local exfoliation of the surface layer of the anterior corneal epithelium was seen (Fig. 1B), and the basal layer exhibited changes in cell shape, nuclear pyknosis, with cell hypertrophy and clarified cytoplasm in, and exfoliation of, individual basal layer cells from the basement membrane. Apoptosis was seen in individual epithelial cells of the intermediate layer, which appeared as a crescent-shaped condensation of nuclear chromatin. The corneal stroma showed edema, delamination of collagen lamellae (Fig. 1B) and lymphocyte infiltration (especially at the site of the expanded venous sinus). Moreover, local endothelial exfoliation was seen.
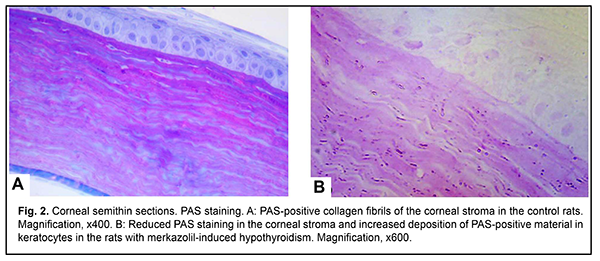
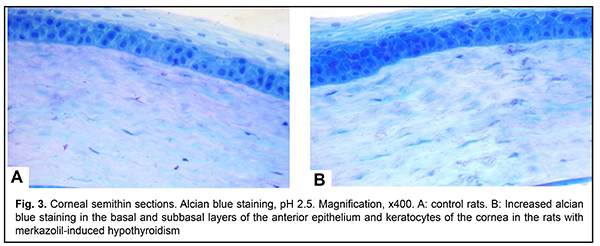
A corneal injury in the presence of EOP has been also diagnosed in the study by Pashkovska [4]. Similar to our study, stromal edema and delamination of collagen lamellae was observed in the rabbits with merkazolil-induced hypothyroidism in the study by Savchuk et al [17]. Besides morphological changes in the cornea, we found increased corneal thickness in the experimental rats compared to the control rats (223.03±5.85 ?m vs 198.32±3.32 ?m, р < 0.01). Palpebral conjunctival white cell infiltration and an increased amount of adipose tissue at the orbital bone have been observed in the study by Poliakova et al [1], which is also in agreement with our findings. We found accumulation of PAS-positive material in the corneal stroma of the control rats, whereas their anterior epithelium, endothelium and keratocytes were areactive (Fig. 2A). There was a decreased reaction in the corneal stroma and an increased deposition of PAS-positive material in keratocytes in the rats with merkazolil-induced hypothyroidism (Fig. 2B). Sulfated glycosaminoglycans were identified in the basal and subbasal layers of the anterior epithelium, anterior chamber endothelium, and, to a less extent, in the stromal keratocytes (Fig. 3A). An increased reaction was noted in the basal and subbasal layers of the anterior epithelium and in the stromal keratocytes in the rats with merkazolil-induced hypothyroidism (Fig. 3B). Accumulation of glycosaminoglycans causes corneal edema.
Lectin histochemistry studies revealed binding specificity of lectin receptors to the structural components of the cornea (Table 2).
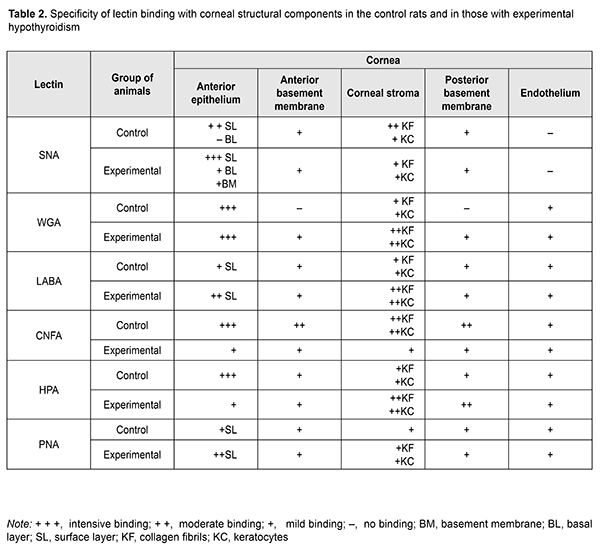
The expression of the receptors of DGalNAc?1-4GlcNAc-specific CNFA lectin was visualized in all the layers of the anterior corneal epithelium and stromal collagen fibrils and keratocytes in the control animals (Fig. 4A). A decreased reactivity of the above lectin to the anterior epithelium and corneal stroma was noted in the experimental rats (Fig. 4B).
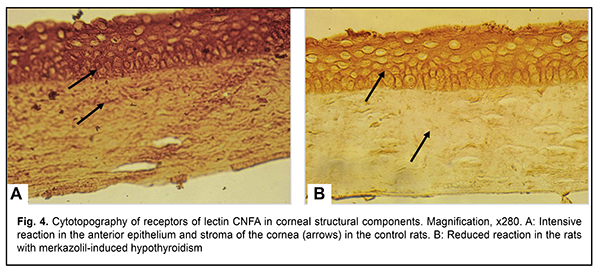
In addition, the binding specificity of WGA lectin to the structural components of the cornea was similar to that of CNFA lectin, with a tendency to decrease in its expression in the stroma; however, we noted an increased expression of the receptors of this lectin in the presence of experimental hypothyroidism, especially at the sites of contact between individual cells of the basal and intermediate layers of the anterior epithelium, and at the surface of stromal keratocytes and collagen fibrils (Figs. 5 A, B).
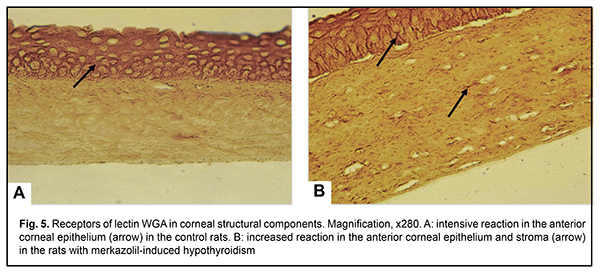
?LFuc-specific lectin LABA exhibited a moderate reactivity to cells of the surface layers of the anterior epithelium, and areactivity in the subbasal and intermediate layers and corneal stroma in the control rats (Fig. 6A). An increased expression of receptors of the above lectin to surface layers of the anterior epithelium, keratocytes and collagen fibrils was noted in the experimental rats (Fig. 6B).
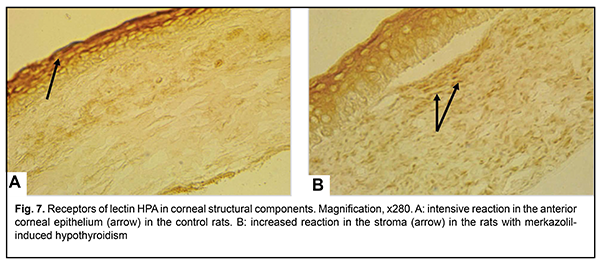
Carbohydrate determinants ?NАcDGal identified by lectin HPA were detected in cells of the anterior corneal epithelium of the control animals in the presence of areactivity of the subbasal layer and corneal stroma (Fig. 7). Reduced carbohydrate determinants ?NАcDGal were observed in the surface layers of the anterior epithelium and basement membrane in the experimental rats, which may be indicative of impaired adhesion between the epithelium and basement membrane at some corneal sites. In addition, there was an increased expression of HPA lectin receptors in the stromal keratocytes and collagen fibrils (Fig. 7B).
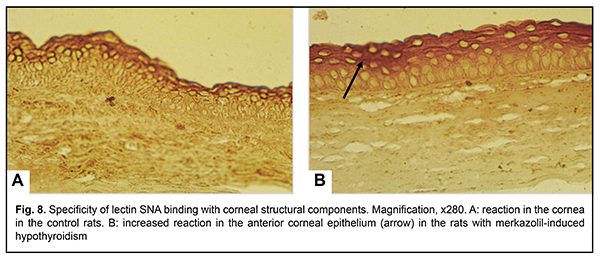
Sialospecific lectin SNA exhibited high affinity for the surface layers of the anterior corneal epithelium and somewhat lower activity to cells of the basal and subbasal layers (Fig. 8A). In the experimental rats, there was an increased expression of the receptors of this lectin at the basement membrane, sites of cell-to-cell contact of the basal and subbasal layers, and cytoplasm of the surface cells of the anterior epithelium, and a reduced expression of the receptors of this lectin at keratocytes and collagen fibrils of the corneal stroma (Fig. 8B). Reduced expression of sialoglycans in keratocytes changes the degree of glycosylation on Golgi apparatus membranes, leading to impaired synthesis of keratin sulfate and corneal crystallines.
Superficial layers of the anterior epithelium and stroma of the cornea of the control animals exhibited insubstantial affinity of glycoconjugates ?DGal (lectin PNA). In the rats with merkazolil-induced hypothyroidism, there was increased affinity of the receptors of this lectin to the superficial layers of the anterior corneal epithelium. Non-active keratocytes have been reported to synthesize corneal crystallines which contribute to maintenance of corneal clarity and ensure optimal refraction similar to those of the lens [11, 18]. They also comprise a portion of the antioxidant defense of the cornea [19]. Keratocytes are activated in injuries or inflammation of various origins [20, 21]. Our finding of polymorphonuclear white cell infiltration of the corneal stroma in experimental hypothyroidism is indicative of the presence of an inflammatory process which may not only cause an injury of the cornea and keratocytes located in this region but also stimulate apoptosis as Wilson et al [21] have indicated previously. Therefore, in the cornea of control animals, there were significant contents of glycopolymers with carbohydrate determinants DGlcNAc, DGal(?1-4)DGlcNA, and NeuNAc(?2-6)DGal (receptors of lectins WGA, CNFA, SNA) in all layers of the anterior epithelium, whereas terminal residues of ?DGalNAc and ?LFuc (receptors of lectins HPA and LABA), in most cases, were found in the superficial layers of the anterior epithelium. In the cornea of animals with experimental hypothyroidism, expression of HPA and CNFA receptors was reduced in the anterior epithelium and increased in keratocytes and collagen fibrils of the corneal stroma, whereas expression of sialoglycans in keratocytes and collagen fibrils of the corneal stroma was increased. Sialoglycan glycome reprogramming may exert effects on epithelial adhesion to the basement membrane and on the corneal clarity which is associated with keratocyte production of crystallines and keratan sulfates. Conclusion The negative impact of merkazolil-induced hypothyroidism on corneal structure was accompanied by (a) keratinization and local exfoliation of the surface layer of the anterior corneal epithelium, (b) edema and lymphocyte infiltration at the site of the expanded venous sinus, (c) local endothelial exfoliation, (d) accumulation of PAS-positive material and sulfated glycosaminoglycans, (e) reduced expression of receptors of HPA (?DGalNАc) and CNFA (GalNAc?1–4GlcNAc) in the anterior epithelium and increased expression of receptors of these lectins in keratocytes and collagen fibrils of the corneal stroma. Redistribution of the latter may exert effects on epithelial adhesion to the basement membrane and change corneal clarity which is associated with keratocyte production of crystallines and keratan sulfates. References 1.Poliakova SI, Bushuieva NN, Kaiali A, Romanenko DV, Shishkina VG. [Determining ocular motility in endocrine ophthalmopathy with the use of the technique of automated analysis of 2D-images of the globe]. Oftalmol Zh. 2014; 6: 53-9. Russian. 2.Hatton MP, Rubin PA. The pathophysiology of thyroid-associated ophthalmopathy. Ophthalmol Clin North Am. 2002 Mar;15(1):113-9. 3.Bhatt R, Nelson CC, Douglas RS. Thyroid-associated orbitopathy: Current insights into the pathophysiology, immunology and management. Saudi J Ophthalmol. 2011; 25(1):15-20. 4.Pashkovska NV. [Endocrine ophthalmopathy in autoimmune disease of the thyroid gland]. Mizhnarodnyi endokrinologichnyi zhurnal. 2014;6(62):169-73. Ukraninan. 5.Fung S, Malhotra R, Selva D. Thyroid orbitopathy. Aust Fam Physician. 2003; 32(8): 615-20. 6.Lutsyk AD, Detiuk ES, Lutsyk MD. [Lectins in histochemistry]. Lviv: Vyshcha shkola; 1989. Russian. 7.Gabius H-J, Kayser K. Introduction to glycopathology: the concept, the tools and the perspectives. Diagn Pathol. 2014;9:4. 8.Hirabayashi J, ed. Lectins. Methods and protocols. New-York: Springer; 2014. 9.Roth J. Lectins for histochemical demonstration of glycans. Histochem Cell Biol. 2011 Aug;136(2):117-30. 10.Rovira VS, Frutos EB, Ferrer C, Hernandez JM, Pastor LM. Identification of apoptotic cells by means of lectin histochemistry: A state of the art review. J Cytol Histol. 2015;6:309. 11.Jester JV. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008; 19(2): 82–93. 12.Antoniuk VO. [Lectins and their sources]. Lviv:KVART; 2005. Ukrainian. 13.Cho EY, Choi HL, Chan FL. Expression pattern of Glycoconjugates in Rat Retina as Analysed by Lectin Histochemistry. Histochem J. 2002;34(11-12):589-600. 14.Shchur MB, Strus KI, Smolkova OV, Yashchenko AM, Lutsyk AD. Modification of carbohydrate determinants of the eyeball structural components on the background of experimental hyperthyroidism according to the lectin histochemistry data. Cell Biol (Henderson, NV). 2017;6(2).136. DOI 10.4172/2324-9293.1000136. 15.Horalskyi LP, Khomych VT, Kononskyi OI. [Basics of histological technique and morphofunctional techniques in health and disease]. Zhytomyr: Polissia; 2005. Ukrainian. 16.Atramentova LA, Utevskaia OM. [Statistical methods in biology]. Gorlovka: Likhtar; 2008. Russian. 17.Savchuk ZL, Klishch IM, Herasymiuk I.Ye. [Changes in corneal structure in rabbits with chemical corneal injury in the presence of merkazolil-induced hypothyroidism]. Journal of Health Sciences. 2014;4(14):190-200. DOI: 10.5281/zenodo.13360. Ukrainian. 18.M?ller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36 (13): 2557–67. 19.Lassen N, Black WJ, Estey T. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008 Apr;19(2):100-12. 20.West-Mays JA, Dwivedі DJ. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–31. 21.Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007 Sep;85(3):305-11.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|