J.ophthalmol.(Ukraine).2019;4:3-7.
|
http://doi.org/10.31288/oftalmolzh2019437 Targeted transscleral laser photocoagulation of the ciliary body in patients with neovascular glaucoma Zadorozhnyy O., Cand Sc (Med); Guzun, O., Cand Sc (Med); Kustryn, T., Cand Sc (Med); Nasinnyk I., Cand Sc (Med); Chechin P., Cand Sc (Med); Korol A., Dr Sc (Med) SI " The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odesa, Ukraine E-mail: laserfilatova@gmail.com TO CITE THIS ARTICLE: Zadorozhnyy O, Guzun O, Kustryn T, Nasinnyk I, Chechin P, Korol A. Targeted transscleral laser photocoagulation of the ciliary body in patients with neovascular glaucoma. J.ophthalmol.(Ukraine).2019;4:3-7. http://doi.org/10.31288/oftalmolzh2019437
Transscleral laser photocoagulation of the ciliary body is used to reduce intraocular pressure (IOP) in neovascular glaucoma, which is associated with a risk of complications. Purpose. To study the efficacy and safety of transscleral laser cyclophotocoagulation in patients with neovascular glaucoma using infrared transillumination to visualize the structures of the ciliary body. Material and Methods. The study included 45 patients (45 eyes) with end- stage neovascular glaucoma. All patients underwent transpalpebral infrared transillumination of the ciliary body in order to accurately position a laser probe during transscleral contact-compression 1064-nm laser cyclophotocoagulation. The follow-up period after the initial treatment was 12 months with monthly control. Results. After 12 months, the mean IOP decreased from 37.7±3.2 to 23.8±5.1 mm Hg (p=0.000). IOP normalization was achieved in 37 patients (82%). The mean number of treatment sessions was 1.8±0.6. During the 12 months follow-up there were no cases of hypotony and phthisis. Conclusion. Infrared transillumination allows imaging of ciliary body structures for targeted positioning a laser probe during transscleral laser cyclophotocoagulation. Targeted transscleral laser 1064-nm cyclophotocoagulation is an effective and safe treatment for patients with neovascular glaucoma. Key words: infrared transillumination, ciliary body, neovascular glaucoma, transscleral cyclocoagulation.
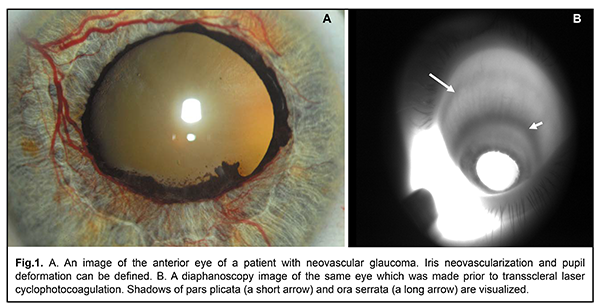
Background Neovascular glaucoma is a severe form of secondary glaucoma which is usually predisposed by retinal ischemia leading to the release of various angiogenic factors and formation of new vessels in the iris and the anterior chamber angle, which results in a stable increase of intraocular pressure [1]. Timely panretinal photocoagulation (once neovascularization in the retina or iris was first seen) resulted in regression of new vessels in 68% and normalization of IOP in 42% of patients [2]. However, despite the treatment, IOP remains elevated in a significant percentage of patients, which can lead to glaucoma progression and complete loss of the visual function [3]. Nowadays, transscleral laser cyclophotocoagulation of the ciliary body is widely used to reduce IOP in neovascular glaucoma. The mechanism of therapeutic action of the procedure consists in reduced production of aqueous by the ciliary body. According to the literature, following transscleral laser photocoagulation of the ciliary body, IOP was successfully reduced in 35-86% of patients with neovascular glaucoma [3-6]. At the same time, there is a high risk of complications associated with transscleral laser cyclophotocoagulation such as vision loss, hyphema, cataract progression, anterior uveitis, hypotony, phthisis, and, very rarely, sympathetic ophthalmia. According to the authors, these are patients with neovascular glaucoma who are at the highest risk of hypotony and phthisis after transscleral laser cyclophotocoagulation [7]. In view of the main trends to perform transscleral laser cyclophotocoagulation of the ciliary body in patients with high visual function, the problem of complication risk reduction in such patients is particularly acute. It is believed that risk of hypotony and phthisis is related with a dose of laser energy delivering during a treatment session [9]. It is also known that there is a problem of accurate localization of the ciliary body structures when performing transscleral cyclophotocoagulation [10]. Thus, use of targeted laser energy delivery to the ciliary body structures, especially in the eyes with neovascular glaucoma, can efficaciously reduce IOP, decrease a laser energy dose during a transscleral cyclophotocoagulation session, and, consequently, reduce the risk of particular complications. Purpose. To study the efficacy and safety of targeted transscleral laser cyclophotocoagulation in patients with neovascular glaucoma using infrared transillumination to visualize the structures of the ciliary body. Material and Methods It was a pilot open-label prospective study. The study was approved by a local Bioethical Committee of the Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine. All the examinees signed the informed consent before being included in the study. Forty-five patients (45 eyes) with neovascular glaucoma were followed up. The patients’ age ranged from 41 to 61. Neovascular glaucoma was secondary to central retinal vein occlusion and branch retinal vein occlusion in 20 patients (20 eyes) and to diabetic retinopathy in 25 patients (25 eyes). At over 1 month before the study, all patients had undergone anterior panretinal laser photocoagulation which did not result in complete regress of new vessels and normalization of IOP. To reduce IOP, all patients received twice-a-day instillations of brimonidine, a carbonic anhydrase and beta-blocker inhibitor in the study eye. All patients underwent examinations in both eyes as follows: best-corrected visual acuity testing, intraocular pressure measurements, anterior eye color imaging, and infrared transpalpebral transillumination. Laser interventions were conducted under epibulbar anesthesia. To this end, a drop of 0.5% proxymethocaine (ALCAINE®, SA Alcon-Couvreur NV, Puurs, Belgium) was instilled thrice with an interval of 10 minutes. In the course of treatment, instillations of indomethacin, a non-steroidal anti-inflammatory agent, (INDOCOLLYRE ophthalmic solution 0.1%, Laboratoire Chauvin SA, France) and parabulbar corticosteroid injections (Dexamethasone, Novo mesto, Slovenia) were also prescribed. Laser treatment included three sessions of transscleral contact-compression laser cyclocoagulation (TSCC LC) using a Neodymium: yttrium-aluminum-garnet (Nd: YAG) laser with ?=1064 nm and with a 600 µm quartz fiberoptic polymer contact probe for dosed compression of the sclera. Impulse laser energy was 0.8 J [11]. A TSCC LC was performed every other day. To position a laser probe accurately during the course of TSCC LC, pars plicata was visualized in all quadrants using infrared transillumination [12]. An infrared transillumination device consisted of a wireless compact LED infrared illuminator with ?=940 nm, a monochrome video camera (Blackfly®, FLIR Integrated Imaging Solutions Inc., Canada) with the possibility of focusing and photo/video recording of a near infra-red signal, and a PC with software for processing the received signal and displaying it on the monitor screen. No local anesthetizing agents were used since the method of illumination was transpalpebral. Shadows of pars plicata and pars plana on the sclera were photo recorded in all quadrants of the eyeball (Figure 1).
The follow-up period lasted twelve months with a once-a-month control visit. Reduction of the IOP rate and regression of the pain syndrome were considered as the primary outcome measure. The secondary outcome measure was a regress of new vessels in the iris and anterior chamber angle and severity and character of complications. Statistical analysis. Means and standard deviations (SD) were calculated for IOP values. The level of significance p ? 0.05 was assumed. Statistical analysis was performed using STATISTICA 10 software. Results Visual acuity in the affected eyes of the patients with neovascular glaucoma corresponded to a perception of light with an inaccurate projection of rays. Baseline IOP was 37.7±3.2 mmHg and 18.9±1.8 mmHg in glaucomatous eyes and intact fellow eyes, respectively (р=0.0001). On the second day after the first TSCC LC session, IOP in glaucomatous eyes decreased to 34.1±2.9, i.e. by 3.6 mmHg as compared to the baseline IOP values; IOP in intact eyes did not change significantly, equaling 19.0±2.0 mmHg (p=0.8). All patients noticed partial or complete regress of pain sensations on the second day after the first session. At 1 month after three TSCC LC sessions, mean IOP in glaucomatous eyes decreased from 37.7±3.2 mmHg to 26.8±2.7 mmHg, i.e. by 10.9 mmHg (29%) as compared to baseline mean IOP. Successful IOP reduction, below 21 mmHg, was achieved in 30 patients (67%). At 1 month, a TSCC LC course was repeated in 15 patients (33%) due to the inadequate compensation of IOP. IOP did not change significantly in intact eyes, equaling 19.0±1.7 mmHg (р=0.16). At the same time, there was a significant difference in IOP between the affected and intact eyes (р=0.000). Best-corrected visual acuity of all studied eyes remained as a perception of light with an inaccurate projection of rays. No complications were recorded during a TSCC LC course and a 1-month postoperative period. At 6 months, mean IOP in the studied eyes decreased by 40.3% and equaled 22.5±3.1 mmHg. With repeat laser interventions in mind, successful IOP reduction, below 21.0 mmHg, was achieved in 38 patients (84%). No complications as hypotony and phthisis were recorded during TSCC LC procedures and a 6-month postoperative period. At 6 months, complete neovascularization regress was in 20%. At 12 months, mean IOP decreased to 23.8±5.1 mmHg as compared to baseline IOP (р=0.000). With repeat laser interventions in mind, successful IOP reduction, below 21.0 mmHg, was achieved in 37 patients (82%). On average, 1.8±0.6 courses of TSCC LC were conducted. No complications as hypotony and phthisis were recorded during TSCC LC procedures and a 12-month postoperative period. Within the follow-up period, 2 cases of hyphema and one case of vitreous hemorrhage were noted. Discussion Fong and colleagues have reported on treatment outcomes in patients with neovascular glaucoma treated with diode laser transscleral cyclophotocoagulation. Complete regress of neovascularization was achieved by the sixth month in 36% of cases. It was possible to reduce IOP in most cases (mean IOP reduction was 33.5 mmHg, 75%) and there was only one eye in which it failed to manage IOP and pain syndrome. However, hypotony and phthisis developed in 27% of cases [13]. In other investigations, high rates of hypotony and phthisis after diode laser transscleral cyclophotocoagulation in patients with neovascular glaucoma were also observed [3, 7, 14]. It has been suggested that in patients with neovascular glaucoma after transscleral cyclophotocoagulation there is a high risk of misbalance between outflow resistance and aqueous production due to a disability to automatically regulate intraocular pressure [7]. In our paper, no cases of hypotony and phthisis were noted in the patients with neovascular glaucoma after transscleral coagulation of the ciliary body during the 12-month follow-up. We suppose that it can be due to a decreased dose of laser energy which was used during a transscleral cyclophotocoagulation session. A wavelength of the infrared laser radiation, being used during transscleral cyclophotocoagulation, is a factor which lowers energy load. We used TSCC LC of the ciliary body using an Nd-YAG laser with ?=1 064 nm. Indeed, it was in 1991 when Vogel and colleagues studied optical properties of the human sclera for various laser wavelengths (442 nm, 633 nm, 804 nm, and 1064 nm) and showed that the highest scleral transmission was at 1064 nm. And contact to the sclera and dosed compression increased scleral transmission of the laser light [19, 20]. Impulse laser energy of 2.5-4.5 J is considered necessary for effective diode laser transscleral cyclophotocoagulation of the ciliary body [7]. Impulse laser energy on our study was 0.8 J. Another way to lower energy load on the anterior eye structures is to position a laser probe accurately in the projection of pars plicata since it is known that morphometric parameters of the choroid are individual and dependant, for example, on axial length [15-17]. Thus, transscleral laser photocoagulation of the ciliary body in the absence of qualitative individual visualization of the ciliary structures can affect the efficacy of the treatment. Dimensions of the ciliary body can be effectively assessed by ultrasound investigation as well as by diaphanoscopy. Ultrasound investigation makes it possible to detect ciliary processes and their position, dimensions, and shape. However, this method does not allow defining a projection of the ciliary body structures on the ocular surface [18]. Transillumination, in contrast, makes it possible to visualize a shadow of the ciliary body to the sclera and to estimate a width of ciliary body structures. To trans-illuminate an eyeball in a visible light range it is necessary to use transcorneal or transscleral illumination. Transpalpebral near-infrared transillumination easily and noninvasively visualizes the ciliary body and accurately estimates the projection of ciliary body structures to the sclera [12]. 360-degree visualization of pars plicata to the sclera makes it possible to accurately position a laser probe on the sclera when performing laser interventions. Rotchford and colleagues have demonstrated in a retrospective study that diode laser cyclophotocoagulation can be used for glaucoma in patients with good visual acuity. The authors used transillumination to define the position of the ciliary body in the presence of congenital glaucoma, high myopia, and limbal alterations. In that study, IOP was controlled in 79.6% of cases with 1.73 treatment sessions, averagely, and no cases of hypotony [8], which is consistent with our findings. Thus, targeted transscleral 1064 nm laser cyclophotocoagulation effectively lowers IOP, decreases energy load to anterior eye structures during treatment, and, respectively, decreases rates of such complications as hypotony and phthisis in patients with neovascular glaucoma. To conclude, firstly, transpalpebral near-infrared transillumination makes it possible to visualize ciliary body structures in patients with neovascular glaucoma and to accurately position a laser probe in the projection of pars plicata while performing transscleral laser cyclophotocoagulation. Secondly, targeted transscleral 1064 nm laser cyclophotocoagulation is an effective and safe treatment for glaucoma. IOP was lowered in 82% of cases with no cases of hypotony and phthisis within a 12-month follow-up period.
References 1.Havens SJ, Gulati V. Neovascular Glaucoma. Dev. Ophthalmol. 2016;55:196-204. 2.Ohnishi Y, Ishibashi T, Sagawa T. Fluorescein gonioangiography in diabetic neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 1994;232:199–204. 3.Nabili S, Kirkness CM. Trans-scleral diode laser cyclophoto-coagulation in the treatment of diabetic neovascular glaucoma. Eye. 2004;18(4):352–6. 4.Oguri A, Takahashi E, Tomita G et al. Transscleral cyclophotocoagulation with the diode laser for neovascular glaucoma. A. Oguri. Ophthalmic Surg. Lasers. 1998;29 (9):722–7. 5.Delgado MF, Dickens CJ, Iwach AG et al. Long-term results of noncontact neodymium:yttrium-aluminum-garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology. 2003;110 (5):895–99. 6.Choy BNK, Lai JSM, Yeung JCC et al. Randomized comparative trial of diode laser transscleral cyclophotocoagulation versus Ahmed glaucoma valve for neovascular glaucoma in Chinese - a pilot study. Clin. Ophthalmol. 2018;12:2545-52. 7.Ishida K. Update on results and complications of cyclophotocoagulation. Curr. Opin. Ophthalmol. 2013;24 (2):102-10. 8.Rotchford AP, Jayasawal R, Madhusudhan S et al. Transscleral diode laser cycloablation in patients with good vision. Br. J. Ophthalmol. 2010;94 (9):1180-3. 9.Vernon SA, Koppens JM, Meno GJ et al. Diode laser cycloablation in adult glaucoma: long-term results of a standard protocol and review of current literature. Exp. Ophthalmol. 2006;34:411–20. 10.Schubert HD. Cyclophotocoagulation: how far posterior to the limbus is the ciliary body? Ophthalmology. 1989;96 (1):139-40. 11.Chechin PP, Guzun OV, Khramenko NI, Peretyagin OA. Efficacy of transscleral Nd:YAG laser cyclophotocoagulation and changes in blood circulation in the eye of patients with absolute glaucoma. J.ophthalmol.(Ukraine).2018;2:34-39. 12.Zadorozhnyy O, Korol A, Nevska A et al. Сiliary body imaging with transpalpebral near-infrared transillumination (Pilot study). Klinika oczna. 2016;3:184-6. 13.Fong AW, Lee GA, O'Rourke P et al. Management of neovascular glaucoma with transscleral cyclophotocoagulation with diode laser alone versus combination transscleral cyclophotocoagulation with diode laser and intravitreal bevacizumab. Clin. Exp. Ophthalmol. 2011;39 (4):318-23. 14.Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br. J. Ophthalmol. 2007;91:1631– 5. 15.Wei WB, Xu L, Jonas JB et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–80. 16.Hairston RJ, Maguire AM, Vitale S. Morphometric analysis of pars plana development in humans. Retina. 1997;17 (2):135-8. 17.Oliveira C, Tello C, Liebmann JM. Ciliary body thickness increases with increasing axial myopia. Am. J. Ophthalmol. 2005;140 (2):324-5. 18.Wang Z, Chung C., Lin J. Quantitative Measurements of the Ciliary Body in Eyes with Acute Primary-Angle Closure. Invest. Ophthalmol. Vis. Sci. 2016;57:3299-305. 19.Vogel A, Dlugos C, Nuffer R et al. Optical properties of human sclera and their significance for trans-scleral laser use. Fortschr. Ophthalmol. 1991;88(6):754-61. 20.Linnik LA, Privalov AP, Chechin PP, Zheltov GI, Tversko? IuL.Laser transscleral contact-compression coagulation of the fundus oculi tissues. Oftalmol Zh. 1989;(6):362-4. In Russian The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|