J.ophthalmol.(Ukraine).2019;3:41-44.
|
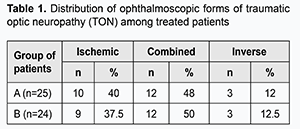
Received: 28 December 2018; Published on-line: 27 June 2019 http://doi.org/10.31288/oftalmolzh201934144 Features of the application of neuroprotective therapy for traumatic optic neuropathy in the clinical setting N.M. Moyseyenko, Cand Sc (Med) Ivano-Frankivsk National Medical University; Ivano-Frankivsk (Ukraine) E-mail: natalymoyseenko@gmail.com TO CITE THIS ARTICLE: Moyseyenko NM. Features of the application of neuroprotective therapy for traumatic optic neuropathy in the clinical setting. J.ophthalmol.(Ukraine).2019;3:41-4. http://doi.org/10.31288/oftalmolzh201934144 Background: The features of the application of neuroprotective therapy for traumatic optic neuropathy (TON) in the clinical setting are not known. Methods: Forty-nine TON patients (49 eyes) with MRI evidence of no compressive optic nerve injury were divided into group A (25 patients) and group B (24 patients) and underwent treatment. Patients of group A were treated with 1000 mg of pre-intravenous methylprednisolone daily for 3 days with subsequent gradual tapering of the dose. Patients of group B were treated with a 1000-mg methylprednisolone infusion daily for 3 days and bilateral electric phosphene stimulation (EPS) for 10 days. Results: We demonstrated in the clinical setting that, compared to steroid-only treatment, steroids plus EPS treatment for TON is characterized by greater improvements in (a) visual acuity, (b) selectivity of action depending on the ophthalmoscopic form of TON, and (c) atrophic processes in the axial bundle of the optic nerve. After combination treatment for TON, visual acuity improved by 0.6 to 0.8, and critical frequency of phosphene disappearance (CFPD) improved by 35 to 45 Hz. Compared with combined or inverse form of TON, ischemic form of TON is seen in the earlier period after the traumatic event. Steroids plus EPS treatment effect was greater in the last form than in the two former forms. Conclusion: Our steroids plus EPS treatment may be an alternative to conventional treatment (megadose steroid-only therapy) for TON in the clinical setting. Keywords: traumatic optic neuropathy, neuroprotective therapy, electric phosphene stimulation Introduction Traumatic optic neuropathy (TON) is an injury to the optic nerve following an episode of head or face trauma and characterized by polymorphic manifestations. Presenting visual acuity is 6/60 or worse in 70% of patients, and 36% of patients may have no light perception [1, 2]. There has been longstanding controversy regarding the best course of treatment for TON. The rate of spontaneous recovery may be as high as 40–60% [3]. Megadose steroids are the mainstay of the conservative treatment of TON. The idea has been adopted from a 30-mg/kg approach to the management of spinal cord injury which demonstrated anti-inflammatory effects. The International Optic Nerve Trauma Study in 1999 was the earliest controlled study of steroids for TON [1, 4]. Although it has been demonstrated that megadose steroid treatment may increase mortality in patients with traumatic brain injury [5], this treatment is widely used in clinical practice in numerous countries [6, 7]. In partial optic atrophy of various etiologies, direct electric stimulation of the optic nerve contributes to restoration of optic nerve function in 70% to 85% of patients [8]. Electric phosphene stimulation (EPS) has been found to lead to a 45% improvement in visual acuity in 87% of patients with partial optic atrophy even after a single application [9]. In addition, it has been found to lead to a 21% to 80% improvement in visual field [10]. Some authors believe that EPS-induced improvements in the blood flow to the eye and brain vision centers are the mechanisms for improvement in visual system activity [11]. The nerve branching induced by an applied electric field has been observed with the subsequent reorganization of the neuronal cytoskeleton [12]. Nevertheless, there is a need for new methods of TON treatment in clinical settings to reduce the systemic toxic effects of corticosteroids and preserve the neuroprotective effects. The purpose of the study was to investigate the application of combination therapy (steroids plus EPS) for neuroprotection in TON in clinical settings. Materials and Methods This prospective study was approved by the Bioethics Committee of the Ivano-Frankivsk National Medical University. The clinical trial stage included treatment of 49 TON patients (49 eyes; men of 20 to 45 years) with MRI evidence of no compressive optic nerve injury. These were divided into group A (25 patients) and group B (24 patients). Patients of group A were treated with 1000 mg of pre-intravenous methylprednisolone daily for 3 days with subsequent gradual tapering of the dose. Patients of group B were treated with a 1000-mg methylprednisolone infusion daily for 3 days and bilateral EPS using “Fosfen” stimulator (with a stimulation current of 3-fold phosphene threshold at the injured side and 300 mA, 10 Hz at the opposite side) for 10 days. According to the proposed classification [13], patients were classified as those with combined, ischemic or inverse form of TON. In both groups, the combined form was the most common (approximately half of all patients), followed by ischemic form and inverse form (12%; Table 1). Spontaneous recovery was observed in patients with edematous form of TON, and they were not subjected to neuroprotective treatment. Optic atrophy of degree 2B was the most common.
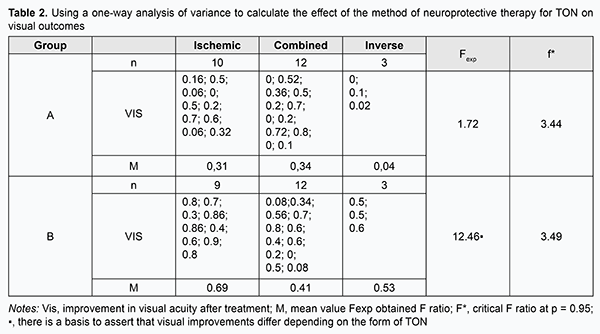
Analysis of variance was used to assess changes in visual acuity, electrically evoked phosphene threshold (EPT) and critical frequency of phosphene disappearance (CFPD) due to significant differences in baseline characteristics (severity of traumatic injury and time from the traumatic event to initiation of treatment). Results A one-way analysis of variance revealed that after treatment, a mean increase in visual acuity in patients with ischemic (n=10), combined (n=12), and inverse (n=12) forms of TON in group A was 0.31, 0.34 and 0.04, respectively. Since the obtained Fexp ratio was smaller than the critical F* ratio at the significance of р=0.95, there was no basis for arguing in favor of the presence of the effect of TON form on steroid-only treatment outcome (Table 2).
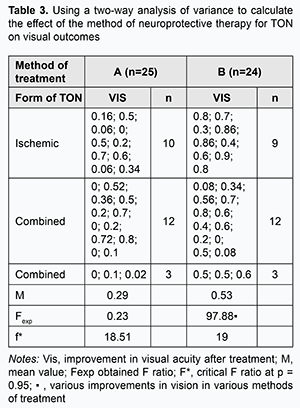
Moreover, a mean increase in visual acuity in patients with ischemic (n=9), combined (n=12), and inverse (n=3) forms of TON in group B was 0.69, 0.41 and 0.53, respectively, and the obtained Fexp ratio was greater than the critical F* ratio at the significance of р=0.95. Therefore, it may be argued that the effect of combination therapy (steroids plus EPS) on the outcome varies depending on the particular form of TON. A two-way analysis of variance revealed that, in group A (n=25), after treatment, the obtained Fexp ratio was smaller than the critical F* ratio at the significance of р=0.95. Therefore, there was no basis for arguing in favor of the presence of a difference in visual outcome after steroid-only treatment between various forms of TON. In addition, in group B (n=24), after treatment, the obtained Fexp ratio was greater than the critical F* ratio at the significance of р=0.95. Therefore, it may be argued that the effect of combination therapy (steroids plus EPS) on the outcome varies depending on the form of TON (Table 3).
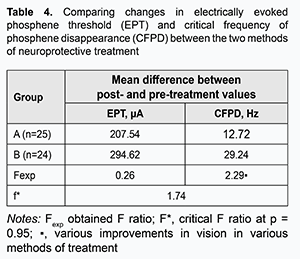
As the mean increase in visual acuity in Group B (0.53) was greater than in Group A (0.29), it may be argued that the effect of steroids plus EPS therapy for TON is greater than that of steroid-only therapy. Moreover, a mean increase in visual acuity in patients with ischemic, combined, and inverse forms of TON after combination therapy (steroids plus EPS) was 0.69, 0.41 and 0.53, respectively. Therefore, combination therapy has a greater effect with regard to improvement of visual acuity in ischemic TON than in combined or inverse forms of TON. The ratio of dependency of the method of treatment on the form of TON is ρ=1.9087/2.8150=0.6785. That is, in 67.85% of the total sample variation, the increase in visual acuity is associated with the method of treatment. A one-way analysis of variance revealed that, after treatment, the mean increase in EPT in Group A (n=25) was 207.54 μA versus 294.62 μA in group B (n=24), and the obtained Fexp ratio was smaller than the critical F* ratio at the significance of р=0.95. Therefore, there was no basis for arguing in favor of the presence of a difference in a degree of restoration of electric sensitivity between the two methods of neuroprotective treatment. In addition, after treatment, the mean increase in CFPD in Group A (n=25) was 12.72 Hz versus 29.24 Hz in group B. As the obtained Fexp ratio was greater than the critical F* ratio at the significance of р=0.95, the combination treatment produced a greater reduction in atrophic processes in the axial bundle of the optic nerve than did the steroid-only treatment (Table 4).
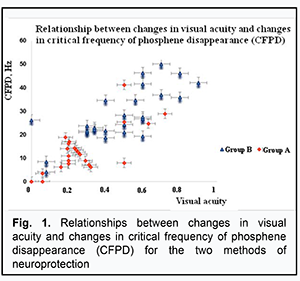
In both groups, there was a direct correlation between the improvement in visual acuity and the increase in CFPD after neuroprotective treatment (r=0.75, p<0.05). Moreover, after neuroprotective treatment, visual acuity and CFPD improved by 0.2-0.4 and 10-20 Hz, respectively, in Group A, and by 0.6-0.8 and 35-45 Hz, respectively, in Group B (Fig. 1), thereby confirming a greater functional efficacy of steroids plus EPS treatment compared to steroid-only treatment.
Discussion Therefore, we demonstrated in the clinical setting that, compared to steroid-only treatment, steroids plus EPS treatment for TON is characterized by greater improvements in (a) visual acuity, (b) selectivity of action depending on the ophthalmoscopic form of TON, and (c) atrophic processes in the axial bundle of the optic nerve. In addition, after combination treatment for TON, an improvement in visual acuity was of 0.6 to 0.8, which is in agreement with the report of improved visual functions after treatment with steroids and EPS for partial optic nerve atrophy [9]. Moreover, after our steroids plus EPS treatment for TON, CFPD improved by 35-45-20 Hz, which reflects a change in the excitability of retinal ganglion cells as well as an increase in the velocity of impulse conduction in the fibers of the optic nerve [14]. Compared with the combined or inverse form of TON, the ischemic form of TON is seen in the earlier period after the traumatic event. Combination treatment effect was greater in the latter form than in the two former forms, which was associated with improved microcirculation after treatment that included EPS [11, 15]. Conclusion
Our steroids plus electric phosphene stimulation treatment may be an alternative to conventional treatment (megadose steroid-only therapy) for TON in the clinical setting. References 1.Ford RL, Lee V, Xing W, Bunce C. A 2-year prospective surveillance of pediatric traumatic optic neuropathy in the United Kingdom. J AAPOS. 2012 Oct;16(5):413-7. 2.Stunkel L, Van Stavern GP. Steroid Treatment of Optic Neuropathies. Asia-Pac J Ophthalmol (Phila). 2018 Jul-Aug;7(4):218-228. 3.Kumaran AM, Sundar G, Lim TC. Traumatic optic neuropathy: a review. Craniomaxillofac Trauma Reconstr. 2015 Mar;8(1):31-41. 4.Yu-Wai-Man P, Griffiths PG. Steroids for traumatic optic neuropathy. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD006032. 5.Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005 Jun 4-10;365(9475):1957-9. 6.Steinsapir KD, Goldberg RA. Traumatic optic neuropathy: An evolving understanding. Am J Ophthalmol. 2011 Jun;151(6):928-933.e2. 7.Yu-Wai-Man P. Traumatic optic neuropathy – clinical features and management issues. Taiwan J Ophthalmol. 2015 Jan-Mar; 5(1): 3–8. 8.Bekhtereva NP. [Curative electric stimulation of the human brain and nerves]. Moscow: AST; 2008. Russian. 9.Drozhenko VS. [Effects of modified electric phosphene stimulation on the function of the visual system in patients with partial optic atrophy]. Thesis for the Degree of Cand Sc (Med). Odessa, 2002. Russian. 10.Eliseeva NM, Serova NK, Gnezditskiĭ VV, Eolchiian SA. [Transdermal electrostimulation of neurosurgical patients with vision disorders]. Vestn Oftalmol. 1997 Jan-Feb;113(1):19-22. Russian. 11.Ponomarchuk VS, Slobodianyk SB, Drozhenko VS. [Comparative analysis of the curative efficacy of electric phosphene stimulation in various eye disorders]. In: [Current issues of ophthalmology: Collection of science works]. Zaporizhzhia; 1997. p.17-21. Russian. 12.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007 Oct 16;69(16):1603-9. 13.Moyseyenko NM. [Prognostic value of ophthalmoscopic symptoms of traumatic optic neuropathy]. International scientific-practical journal “Ophthalmology”. 2017; 1(6): 34-44. 14.Kerns Y, Fakhouri AJ, Weinrib HP, Freeman J.A. Electrical stimulation of nerve regeneration in the rat: the early effects evaluated by a vibrating probe and electron microscopy. Neuroscince. 1991;40(1):93-107. 15.Lee V, Ford RL, Xing W, et al. Surveillance of traumatic optic neuropathy in the UK. Eye (Lond). 2010 Feb;24(2):240-50. doi: 10.1038/eye.2009.79. Crossref PubMed The author certifies that she has no conflicts of interest in the subject matter or materials discussed in this manuscript.
|