J.ophthalmol.(Ukraine).2019;3:14-19.
|
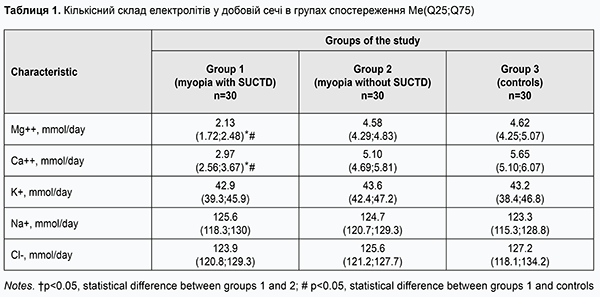
http://doi.org/10.31288/oftalmolzh201931419 Received: 05 March 2019; Published on-line: 27 June 2019 Clinical and diagnostic value of impairments in electrolyte metabolism in children with acquired myopia T.Ye. Tsybulska,1 Cand Sc (Med), S.V. Gorbachova,1 Dr Sc (Biol), T.S. Zavgorodnia,2 Cand Sc (Med) 1 Zaporizhzhia State Medical University; Zaporizhzhia (Ukraine) 2 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) E-mail: tamila.eye@gmail.com TO CITE THIS ARTICLE: Tsybulska TYe, Gorbachova SV, Zavgorodnia TS. Clinical and diagnostic value of impairments in electrolyte metabolism in children with acquired myopia. J.ophthalmol.(Ukraine).2019;3:14-9. http://doi.org/10.31288/oftalmolzh201931419 Background: Identifying the biochemical features of the body in myopia associated with connective tissue dysplasia is still important in the ophthalmologist’s practice. Purpose: To assess the clinical and diagnostic value of impairments in electrolyte merabolism in children with acquired myopia. Methods: Group 1 included 30 children with mild myopia associated with the syndrome of unspecified connective tissue dysplasia (SUCTD), and Group 2 included 30 children with mild myopia without SUCTD. Group 3 (controls) included 30 pediatric healthy controls without ocular disease. Electrolyte balance was assessed by measuring magnesium, calcium, sodium, potassium and chloride ion concentrations in daily urine. Results: Magnesium and calcium ion concentrations in daily urine in children in Group 1 were significantly (2- and 1.7-fold, respectively) lower compared with children in Group 2, and significantly (2.1- and 1.9-fold, respectively) lower compared with controls (р < 0.05). There was no difference in sodium, potassium or chloride ion concentrations in daily urine among study groups. ROC analysis found that an optimum cutoff magnesium ion concentration of ≤2.3 mmol/day in daily urine was 97% sensitive and 80% specific, with an area under curve value (AUC) of 0.97±0.12 (CI 0.95-0.99) (р < 0.0001). In addition, an optimum cutoff calcium ion concentration of ≤3.42 mmol/day in daily urine was 96% sensitive and 85% specific, with an AUC value of 0.94±0.27 (CI 0.88-0.98) (р < 0.0001). Magnesium ion concentration in daily urine was moderately negatively correlated with dysplasia severity (r= -0.65, р < 0.05), and calcium ion concentration was moderately negatively correlated with dysplasia severity (r= -0.59, р < 0.05). Conclusion: The current study determined the diagnostic values of magnesium and calcium ion concentrations (≤ 2.3 mmol/day and ≤ 3.42 mmol/day, respectively) which can be used as biomarkers for laboratory screening for the presence of SUCTD in clinical practice. Keywords: myopia, connective tissue dysplasia, diagnostics, electrolytes, urine, children Introduction Acquired myopia is the most common eye condition in children [1]. Studies on the pathogenesis of myopia have demonstrated that abnormalities in the sclera (the connective tissue tunic of the eye) take place in the presence of a decreased structural integrity of collagen fibrils and are frequently a manifestation of the syndrome of unspecified connective tissue dysplasia (SUCTD) [2, 3]. The prevalence of SUCTD in school-age children has been found to be 74%-85% [2, 4]. The presence and degree of SUCTD are determined by the phenotypic criteria of systemic connective tissue involvement; based on the assessment using these criteria, myopia is a major visceral manifestation of connective tissue dysplasia, with a prevalence varying from 36.2% to 79.4% in children with this disorder [2, 4, 5]. It has been reported that patients with acquired myopia associated with SUCTD demonstrate an early onset of the disease, tend to have a rapid disease progression and develop chorioretinal complications, and show some specific anatomic and optic features of the visual system [6, 7]. Connective tissue metabolism in the acquired myopia in children has been still poorly investigated, although some studies suggest the development of imbalance in the macro- and microelement metabolism and presence of pathological changes in the metabolism of connective tissue in the sclera and the entire body in moderate or severe myopia [2, 8, 9]. The pieces of data available, however, are scarce and contradictory. Unfortunately, to the best of our knowledge, there are no clear criteria for electrolytic metabolism conditions evidencing the presence of connective tissue dysplasia in acquired myopia. Therefore, conducting further research of the biological markers of connective tissue metabolism is reasonable for understanding the pathogenesis of myopia associated with SUCTD. Identifying the biochemical characteristics of the body in myopia associated with SUCTD in children will contribute to improved diagnosis and treatment of patients with this condition. The purpose of the study was to assess the clinical and diagnostic value of impairments in electrolyte metabolism in children with acquired myopia. Materials and Methods Sixty children with mild myopia and 30 pediatric healthy controls without ocular disease underwent examination. Written informed consent was obtained from parents or guardians of all study participants and all investigations were conducted in according with the principles expressed in the Helsinki declaration. Group 1 included 30 children with mild myopia associated with SUCTD, and Group 2 included 30 children with mild myopia without SUCTD. Group 3 (controls) included 30 pediatric healthy controls without ocular disease. There was no significant difference in age between groups, and the mean age varied from 7 to 12 years. Children underwent the following evaluations: uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA), autorefractometry and keratometry before and after cycloplegia, optical biometry (ZEISS IOL Master 500, Germany), and Cirrus spectral domain optical coherence tomography (Cirrus HD-OCT 4000 model; Carl Zeiss Meditec, Inc.). The presence and severity of SUCTD was graded as per the grading system proposed by Milkovska-Dimitrova and Karkashev [4]. Mild SUCTD was found in 17 patients (56.6%), and moderate SUCTD, in 13 patients (43.3%) of Group 1. Electrolyte balance was assessed by measuring magnesium, calcium, sodium, potassium and chloride ion concentrations in daily urine. Sodium, potassium and chlorides levels in urine samples were measured by direct potentiometry using a Prestige 24i Chemistry Analyzer (Tokyo Boeki) with the ISE unit. Magnesium and calcium levels in urine samples were measured using a Prestige 24i Chemistry Analyzer and MG Prestige 24i and Calcium Arsenazo Prestige 24i assays (Cormay, Poland). Statistical analyses were conducted using Statistica 6.0 (StatSoft, Tulsa, OK, USA; License No. AXXR712D833214FAN5) software. Data are presented as median (Me) and interquartile range (Q25; Q75). Group comparisons were performed using the nonparametric Kruskal–Wallis H test. Correlations are presented using Spearman ρ. Receiver operating characteristic (ROC) curve analysis with determination of the area under curve (AUC) and 95% confidence intervals (CI) was performed to assess the relative quality of obtained data. The level of significance p ≤ 0.05 was assumed. Results Table 1 shows urinary electrolyte levels in study groups. Magnesium and calcium ion concentrations in daily urine in children in Group 1 were significantly (2- and 1.7-fold, respectively) lower compared with children in Group 2, and significantly (2.1- and 1.9-fold, respectively) lower compared with controls (р < 0.05). There was no difference in sodium, potassium or chloride ion concentrations in daily urine among study groups.
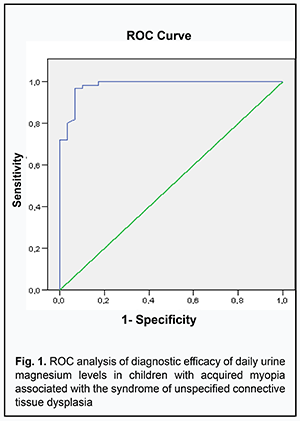
ROC analyses enabled us to show good differentiation between biochemical parameters of children with myopia associated with SUCTD and children with myopia without signs of SUCTD. The optimum cut-off value for urine magnesium levels was ≤2.3 mmol/day (sensitivity: 0.97; specificity: 0.80) (Fig. 1) and the AUC was 0.97±0.12 (CI 0.95-0.99) (р <0.0001) in myopic children with SUCTD. In addition, the optimum cut-off value for urine calcium levels was ≤3.42 mmol/day (sensitivity: 0.96; specificity: 0.85) (Fig. 2) and the AUC was 0.94±0.27 (CI 0.88-0.98) (р <0.0001).
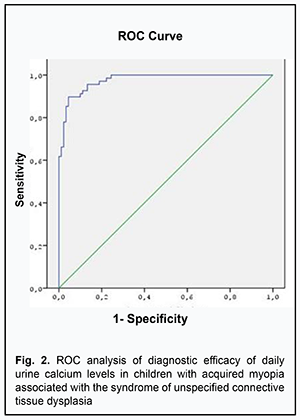
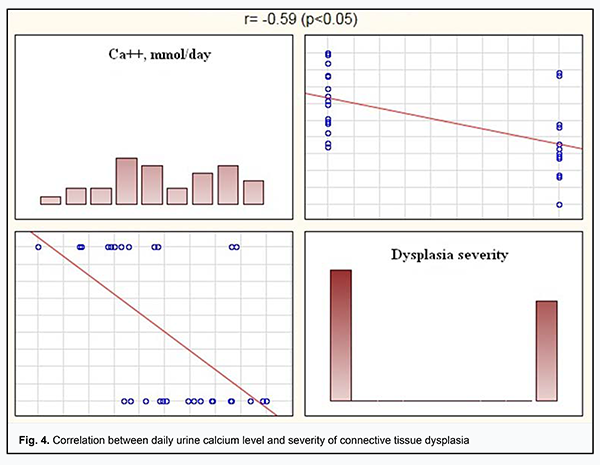
Our ROC analysis findings indicated a high level of diagnostic value of daily urine magnesium and calcium levels as biochemical indicators of connective tissue dysplasia. We conducted Spearman correlation analysis between daily urine electrolyte levels and severity of connective tissue dysplasia. There were significant correlations between daily urine magnesium and calcium levels and dysplasia severity. Thus, daily urine magnesium level was moderately negatively correlated with dysplasia severity (r= -0.65, р <0.05) (Fig.3), and daily urine calcium level was moderately negatively correlated with dysplasia severity (r= -0.59, р <0.05) (Fig.4).
Discussion Studies on proteoglycans, glycoproteins, glycosaminoglycans, oxyproline and expression of proteolytic enzymes in biochemical fluids have addressed the widest range of issues with regard to the biochemical assessment of the connective tissue in myopia. Several investigators have demonstrated increased serum levels of carboxypropeptide of type 1 procollagen (P1CP) and human cartilage glycoprotein 39 (HC-gp39) in progressive myopia [10]. Others have reported on elevated plasma levels of hyaluronidase, antitrypsin, free oxyproline, and total amine nitrogen in children with progressive myopia [8, 9], indicating impairment in activity of the enzyme complex that influences collagen synthesis and breakdown. A supply of the ocular tissues and entire body with microelements is an important factor influencing the genesis of myopic refractive errors, since such microelements as magnesium, calcium, potassium and others contribute to the synthesis of connective tissue proteins. Heterogeneous findings have been reported on this subject. In the stjudy by Kovalenko and Yakovleva [11], high myopes demonstrated elevated blood potassium and chlorine levels and low blood calcium levels. Budnik [2] has reported that serum magnesium and calcium levels in myopic children were practically within the normal age limits (0.82±0.06 mmol/l against the norm of 0.8-1.0 mmol/l and 2.3±0.3 mmol/l against the norm of 2.3-2.75 mmol/l, respectively). Changes in not only systemic, but also local levels of microelements in myopia have been demonstrated. The histochemical analysis of myopic ocular tissues showed significantly decreased levels of selenium, zinc and magnesium [3]. In the current study, daily urine was used as a biological fluid. Blood electrolyte levels do not always reflect the intracellular deficiency of relevant electrolytes in a body tissue [12]. In addition, urine study is a convenient non-invasive diagnostic technique allowing for personalized patient management which is important in pediatric patients. We found that daily urine magnesium and calcium levels in children with acquired myopia associated with connective tissue dysplasia were significantly (2- and 1.7-fold, respectively) lower compared with children with acquired myopia without connective tissue dysplasia. However, we found no difference in blood potassium, sodium or chlorine levels between study patients and controls. Moreover, we found correlations between daily urine magnesium and calcium levels and dysplasia severity. This is in agreement with previous reports on macro- and microelement profiles for children with connective tissue dysplasia which indicated that these elements influence the connective tissue metabolism, and, specifically, magnesium deficiency is a trigger for connective tissue dysplasia [4, 13]. The current study determined the diagnostic values of daily urine magnesium (≤ 2.3 mmol/day) and calcium (≤ 3.42 mmol/day) levels which can add to the knowledge base of the biochemical processes in the connective tissue structures of the body and be used as biomarkers for laboratory screening for the presence of SUCTD in clinical practice. The obtained data indicate the need for a differential approach to managing myopia which is pathogenetically based on individual assessment of electrolyte levels in a particular child with acquired myopia. Magnesium and calcium function as cofactors in biochemical reactions in intra- and extracellular maturation of collagen and other structural elements of the connective tissue, and, therefore, it is deemed reasonable to integrate magnesium and calcium-containing medications into the treatment of the disease. Conclusion First, magnesium and calcium ion concentrations in daily urine in children with acquired myopia associated with SUCTD were significantly (2- and 1.7-fold, respectively) lower compared with children with acquired myopia without SUCTD, and significantly (2.1- and 1.9-fold, respectively) lower compared with controls (р < 0.05). Second, determination of magnesium and calcium ion concentrations of ≤ 2.3 mmol/day and ≤ 3.42 mmol/day, respectively (sensitivity: 0.97 and 0.96, respectively; specificity: 0.80 and 0.85, respectively) in daily urine in children with acquired myopia allows for diagnosing the SUCTD.
Finally, magnesium ion concentration in daily urine was moderately negatively correlated with dysplasia severity (r= -0.65, р < 0.05), and calcium ion concentration was moderately negatively correlated with dysplasia severity (r= -0.59, р < 0.05). References 1.Moiseienko EO, Golubchikov MV, Mykhalchuk VM, Rykov SO. [Ophthalmological care in Ukraine in 2014-2017. Analytical and statistical reference book]. Kyiv: Logos; 2018. Ukrainian. 2.Budnik TV. [A comparison of phenotypic and clinical signs of connective tissue dysplasia, micronutrient sufficiency and ophthalmic data in children with progressive myopia]. Perinatologiia I pediatriia. 2014;2:41–5. Russian. 3.Iomdina EN, Tarutta EP. [Modern trends of basic research in pathogenesis of progressive myopia]. Vestn Ross Akad Med Nauk. 2014;(3-4):44–9. Review. Russian. 4.Kadurina TI, Gorbunova VN, editors. [Displasia of the Connective Tissue]. St Petersburg: ELBI; 2009. Russian. 5.Li VV, Smoliakovs GP, Egorov VV, Kashura OI. [Relevance of the Problem of Myopia in School-age Children with Signs of Undifferentiated Connective Tissue Dysplasia]. Ophthalmology in Russia. 2018;2S:58–64. Russian. 6.Seleznev AV, Nasu H. [Dynamics of myopia in individuals with syndrome of connective tissue dysplasia]. Oftalmokhirurgiya. 2012; 4:73. Russian. 7.Tsybulskaya TYe, Zavgorodnia NG, Ivachnenko OM, Pashkova OYe. Anatomical, optical, biomechanical and morphometric parameters of the eye in children with acquired myopia and syndrome of undifferentiated connective tissue dysplasia. Zaporozhye medical journal. 2018; 3(108):392–6. 8.Vinetskaia MI, Boltaeva ZK, Iomdina EN, Andreeva LD. [Biochemical aspects of progressive myopia]. Oftalmol Zh. 1988;(3):155–8. Russian. 9.Bushueva NN. [Current aspects of the pathogenesis and treatment of progressive myopia]. In: [Proceedings of the Ukrainian National Conference of Pediatric Ophthalmology]. 4-5 October, 2012, Sevastopol. p.281–-91. Russian. 10.Bikbov MM, Dautova ZA, Samatova RR, et al. [Certain biochemical blood parameters in children with acquired myopia]. Vestnyk Ros Voenno-Meditsinskoi akademii. 2009; 3:52–4. Russian. 11.Kovalenko VV Iakovleva AN. [Biochemical indices of the blood in schoolchildren with myopia]. Oftalmol Zh. 1978;33(4):284–6. Russian. 12.Gromova OA, Kalacheva AG, Torshin IYu, et al. [On the diagnostics of magnesium deficiency]. Arkhiv’’ vnutrennei meditsiny. 2014;3(17):6–10. Russian. 13.Torshin IYu, Gromova OA. [Molecular mechanisms of connective tissue dysplasia and those underlying the action of magnesium]. Rossiiskii meditsinskii zhurnal. 2008;4:263–9. Russian. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|