J.ophthalmol.(Ukraine).2019;3:9-13.
|
http://doi.org/10.31288/oftalmolzh20193913 Received: 21 January 2019; Published on-line: 27 June 2019 Serum VEGF levels in infants at risk for developing retinopathy of prematurity S.V. Katsan, Cand Sc (Med); S.G. Fedotova, Ophthalmologist Filatov Institute of Eye Diseases and Tissue Therapy; Odesa (Ukraine) E-mail: adakhovskayaa@gmail.com TO CITE THIS ARTICLE: Katsan SV, Fedotova SG. Serum VEGF levels in infants at risk for developing retinopathy of prematurity. J.ophthalmol.(Ukraine).2019;3:9-13. http://doi.org/10.31288/oftalmolzh20193913 Background: Because excessive growth of abnormal retinal vessels in the developing eye of the premature infant underlies retinopathy of prematurity (ROP), it may be hypothesized that a change in vascular endothelial growth factor (VEGF) level in the infant’s vascular bed predicts the course of the disease. Purpose: To determine serum VEGF levels and to assess changes in these levels over time in infants at risk for developing ROP. Materials and Methods: Twenty three infants born preterm underwent examinations for ROP. Two examinations were performed 14±3 days apart. A 1-ml blood sample was collected to obtain a 0.5-ml serum sample from each infant. Serum was frozen at -20° С until analysis. Serum VEGF levels were assessed by enzyme-linked immunosorbent assay (ELISA) method using Human VEGF-A ELISA Kit (Ray Biotech Inc., Norcross, GA) following the manufacturer’s instructions. Newborns were divided into two groups: those who exhibited ROP progression or absence of improvement (Group 1), and those who exhibited regression of ROP (Group 2). A Student’s t-test or Wilcoxon rank-sum test was used for two-group comparison. Results: There were no significant differences between groups with regard to birth weight or gestational age (p<0.17; p<0.06). In addition, there was no significant difference with regard to serum VEGF level between groups at the first and second screening examinations (p<0.56). The difference in serum VEGF levels between two screening examinations for Group 1 was not significant (Friedman test, p = 0.27), whereas a decrease in serum VEGF levels between these examinations for Group 2 was significant (Friedman test, p = 0.046). Conclusion: There was no significant difference with regard to serum VEGF level between groups at the first and second screening examinations. The increase in serum VEGF level between two examinations for infants who exhibited ROP progression or absence of improvement was not statistically significant. Infants with disease regression demonstrated a significant decrease in serum VEGF level at the second examination compared to the first examination. Keywords: retinopathy of prematurity, VEGF
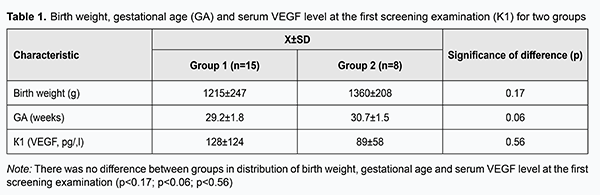
Introduction Retinopathy of prematurity (ROP) is a severe eye disorder associated with immaturity of retinal vessels in children born preterm. Although, in the majority of cases, ROP regresses spontaneously, only a timely intervention can prevent imminent blindness in a neonate with progressive ROP. Vacularization of the human retina begins at 14 weeks gestational age, and is completed by approximately 40 weeks, at term [1]. Vascular endothelial growth factor (VEGF) plays a crucial role in the development of all human organs beginning from the fetal period of development. A positive role of VEGF in this process has been many times demonstrated in animal models and in humans [2-5]. In addition, this factor plays an important role in pathologic angiogenesis which is associated with a variety of eye conditions including diabetic proliferative retinopathy, neovascular subretinal membrane, neovascular glaucoma and ROP. Because excessive growth of abnormal retinal vessels in the developing eye of the premature infant underlies ROP, it may be hypothesized that a change in VEGF level in the infant’s vascular bed predicts the course of the disease. The purpose of the study was to determine serum VEGF levels and to assess changes in these levels over time in infants at risk for developing ROP. Materials and Methods Twenty three preterm neonates (≤ 32 weeks of gestation with a birth weight ≤1500 g) without severe systemic disorders or developmental abnormalities underwent serum VEGF level assessment. Screening started at postnatal age 4th week and continued until complete vascularization of the retina or until sub-threshold or more severe ROP developed requiring for surgical intervention. Dilated fundus examination was performed with a binocular direct ophthalmoscope and Panocam (Visunex Medical Systems, Fremont, CA). A 1-ml blood sample was collected to obtain a 0.5-ml serum sample from each infant. Two examinations were performed 14±3 days apart. It should be noted that the current study did not need for additional invasive interventions since we used the blood doses for routine blood tests. Serum was frozen at -20° С until analysis. Serum VEGF levels were assessed by enzyme-linked immunosorbent assay (ELISA) method using Human VEGF-A ELISA Kit (Ray Biotech Inc., Norcross, GA) following the manufacturer’s instructions. Newborns were divided into two groups: those who exhibited ROP progression or absence of improvement (Group 1), and those who exhibited regression of ROP (Group 2). Student’s t-test was used for group comparison for normally distributed variables and Wilcoxon rank-sum (Mann-Whitney) test was used for group comparison for non-normally distributed variables. Results Of the 23 study infants, there were 13 boys and 10 girls. Gestational age at birth ranged from 26 weeks to 32 weeks (mean ± SD, 29±3 weeks), and birth weight ranged from 880 g to 1,600 g (mean ± SD, 1,240±360 g). At the first examination, ROP was found in 8 infants, and avascular retinal areas were found in 15 infants. Fundus changes were assessed at the second examination. ROP progressed, regressed spontaneously, or remained stable in 2, 3 and 3 infants, respectively. In addition, avascular retinal areas improved (to normal retinal vascularization), worsened (due to development of ROP) or remained stable in 5, 4 and 6 infants, respectively. Group 1 (progressive or stable ROP) involved 15 infants, and Group 2 (regressive ROP) involved 8 infants. First, the groups were compared with regard to birth weight, gestational age and serum VEGF level at the first screening examination (Table 1). There were no significant differences between groups with regard to birth weight, gestational age or serum VEGF level.
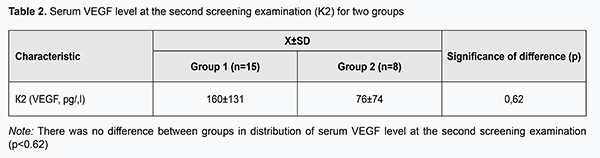
Second, the groups were compared with regard to longitudinal serum VEGF levels. Table 2 presents serum VEGF levels for the groups at the second screening examination (14±3 days after the first screening examination). There was no significant difference between groups with regard to serum VEGF level at this time point (p = 0.62). Friedman test was used to analyze longitudinal change in serum VEGF levels.
We calculated differences in serum VEGF levels between the two examinations for those who exhibited ROP progression or absence of improvement (Group 1), and those who exhibited regression of ROP (Group 2). The difference in serum VEGF levels between the two examinations for those who exhibited regression of ROP (р=0.046), but not for those who exhibited ROP progression or absence of improvement (p = 0.27), was significant. Discussion ROP pathogenesis has been linked with a change in VEGF in the vascular bed. The role of VEGF in the formation of ROP has been demonstrated in the mouse models of oxygen-induced ROP [6]. The initial formation of the human fetal retinal vasculature takes place under favorable conditions with relatively stable oxygenation (so called physiological hypoxia), but a baby exhibits oxidative stress after preterm birth [5, 7]. At that point hyperoxygenation substantially inhibits VEGF release which, in turn, arrests normal retinal vasculogenesis. As a result, avascular areas form in the retina. Because the absence of vessels in the periphery of the eye results in hypoxia of these areas, this tissue condition is the stimulus for excessive VEGF production and uncontrolled neovascularization. VEGF has been shown to be a major promoter of neovascularization [5, 8, 9], although other regulating factors (Insulin-like Growth Factor 1 (IGF-1), IL-1, IL-6, etc.) also play a role. This finding has provided a basis for the use of anti-VEGF agents in the therapy of ROP. Although there is no general consensus on whether the benefits outweigh potential harm for preterm newborns, the efficacy of anti-VEGF agents in the therapy of ROP has been demonstrated. Given the role of VEGF in the development of ROP, numerous studies were conducted to determine a normal range for the VEGF level in healthy, term neonates. Attention was also given to umbilical cord serum VEGF levels and to the potential for using them as a predictor of ROP. Prior studies examining VEGF concentrations at birth have detected no difference in VEGF concentrations in cord blood between preterm and term infants [10], or between preterm infants who later developed ROP and those who did not develop this disease [11]. A significant difference in blood VEGF levels, however, has been found between neonates and healthy adults [12]. Therefore, the issue of determining a normal range for the VEGF level in human biological media is still to be solved. Until recent years, there have been no reports on a normal range for the VEGF level in neonates. It should be noted that increased serum VEGF levels have been demonstrated in studies on other pathological conditions (such as respiratory or cardiovascular developmental disorders) in preterm infants. There have been several reports on studies on serum VEGF levels in neonates. Ozlem Teksam et al [13] compared infants with meconium stained amniotic fluid (MSAF) with healthy term newborn infants without MSAF with regard to VEGF levels in cord and arterial blood. Although they did not find significant differences in these indices between the two groups, the results of the study were interesting in that the authors determined blood VEGF levels for healthy term newborns. They found that plasma VEGF levels in the MSAF group of 18 newborns were 190 pg/ml (range, 35 to 1100 pg/ml) versus 155 pg/ml (range, 31 to 360 pg/ml) in the control group of 16 healthy newborns. Tsao et al [14] compared cord blood VEGF concentrations in preterm infants with respiratory distress syndrome (RDS) versus infants without RDS. They found that infants with RDS had significantly lower cord blood VEGF levels. It should be noted that the risk factors for developing RDS (a lower gestational age, lower birth weight, higher incidence of mechanical ventilation requirements, longer duration of mechanical ventilation, and lower Apgar scores at 1 and 5 min) were the same as for ROP. Hellgren et al [10] determined VEGF plasma concentrations in cord blood and longitudinal serum VEGF concentrations for preterm infants in relation to ROP. The study included 52 infants born at <31 week gestational age. Thirty-three infants were classified as non-ROP, 10 infants were classified as having nonproliferative ROP, and 9 infants were classified as having proliferative ROP and were later treated with laser for ROP. VEGF concentrations were analyzed in blood samples collected at birth, at 3 days postnatal age, and then weekly until at least a gestational age of 35 weeks. No correlations were found between VEGF concentrations at birth and later ROP status; median (95% CI for median) concentrations in cord blood were 20.2 (7.2–36.1) pg/ml, 32.4 (2.1–73.1) pg/ml, and 29.6 (6.2–89.8) pg/ml in infants with no ROP, nonproliferative ROP, and treated for ROP, respectively. However, infants requiring treatment for ROP had significantly higher median VEGF serum concentrations at 34, 35, and 36 week PMA (the time points corresponding to active retinal proliferation) than infants without ROP [15]. A study by Australian researchers reported on a normal range for the serum VEGF level in healthy, term neonates [16]. The serum VEGF-A levels were obtained from 32 neonates born at term infants (16 males and 16 females). The mean postnatal age for these infants at the time of sampling was 6.9 (4.3) days. There was no significant difference in serum VEGF-A level between female and male infants. There was no correlation between birth weight and plasma VEGF-A levels. There was also no significant correlation between time of sampling and VEGF-A level. The median sVEGF-A level was 976 (394 – 1,635) pg/mL. As we can see, studies vary widely with regard to findings for ranges of serum VEGF levels, and there are, to the best of our knowledge, no regulations regarding these ranges. That is why each study compares the study group with the control group with regard to ranges of these levels. In the current study, we found no statistically significant difference in serum VEGF levels among two groups and between examinations, although the ranges of these levels were wide. It was, however, interesting that infants with disease regression demonstrated a significant decrease (р=0.046) in serum VEGF level at the second (76 ± 74 pg/ml) compared to the first examination (89 ± 58 pg/ml). Before this study we believed that there is a close relationship between an increase in VEGF level and progression of ROP. Findings of the current study, however, made us approach the problem from a different angle. A reduction in or normalization of VEGF level might indicate a stabilization or even regression of the disease. A decline in serum VEGF level in the presence of retinal improvement in infants at risk for developing ROP also advocates a relationship between this characteristic and the course of ROP. There is, therefore, a need for further research and new methods for diagnosing ROP. Conclusion Infants who exhibited ROP progression or absence of improvement had an increased serum VEGF level at the second screening examination compared to the first screening examination (р=0.27). Infants with disease regression demonstrated a significant decrease (р=0.046) in serum VEGF level at the second examination (76 ± 74 pg/ml) compared to the first examination (89 ± 58 pg/ml). References 1.Saidasheva EI, Somov EE, Fomina NV. [Selected lectures on neonatal ophthalmology]. St. Petersburg: Nestor Istoriya; 2006. Russian. 2.Fierson WM, American academy of pediatrics Section on Ophthalmology, American academy of ophthalmology, American association for pediatric ophthalmology and strabismus, American association of certified orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2018 Dec;142(6). pii: e20183061. 3.Jefferies AL, Canadian Paediatric Society, Fetus and Newborn Committee. Retinopathy of prematurity: Recommendations for screening. Paediatr Child Health. 2010 Dec; 15(10): 667–70. 4.Tasman W. Retinopathy of prematurity: Do we still have a problem?: The Charles L. Schepens lecture. Arch Ophthalmol. 2011 Aug;129(8):1083-6. 5.Verma A, Nema N, Patel S, Ishrat S, Sidharth M. Retinopathy of Prematurity: Incidence and Risk Factors Research Article. Int J Ophthalmol Eye Sci. 2016;4:198–201. 6.Royal College of Paediatrics and Child Health. Guideline for the Screening and Treatment of Retinopathy of Prematurity. 2010. 7.Taqui AM, Syed R, Chaudhry TA, Ahmad K, Salat MS. Retinopathy of prematurity: frequency and risk factors in a tertiary care hospital in Karachi, Pakistan. J Pak Med Assoc. 2008;58:186–90. 8.Abdel HA, Mohamed GB, Othman MF. Retinopathy of Prematurity: A Study of Incidence and Risk Factors in NICU of Al-Minya University Hospital in Egypt. J Clin Neonatol. 2012 Apr;1(2):76-81. doi: 10.4103/2249-4847.96755. 9.Visser Kift E, Freeman N, Cook C, Myer L. Retinopathy of prematurity screening criteria and workload implications at Tygerberg Children's Hospital, South Africa: A cross-sectional study. S Afr Med J. 2016 May 12;106(6). 10.Hellgren G, Löfqvist C, Hård AL, et al. Serum concentrations of vascular endothelial growth factor in relation to retinopathy of prematurity. Pediat Res. 2016 Jan;79(1-1):70-5. 11.Galazios G, Papazoglou D, Giagloglou K, Vassaras G, Koutlaki N, Maltezos E. Umbilical cord serum vascular endothelial growth factor (VEGF) levels in normal pregnancies and in pregnancies complicated by preterm delivery or pre-eclampsia. Int J Gynaecol Obstet. 2004 Apr;85(1):6-11. 12.Malamitsi-Puchner A, Tziotis J, Tsonou A, Protonotariou E, Sarandakou A, Creatsas G. Changes in serum levels of vascular endothelial growth factor in males and females throughout life. J Soc Gynecol Investig. 2000 Sep-Oct;7(5):309-12. 13.Teksam O, Tekinalp G, Yurdakok M, Yigit S, Korkmaz A. Dicle G. Vascular Endothelial Growth Factor Levels in Newborns with Meconium Stained Amniotic Fluid. Indian J Pediatr. 2008 Oct;75(10):1015-7. doi: 10.1007/s12098-008-0174-7. 14.Tsao PN, Wei SC, Chou РС, et al. Vascular Endothelial Growth Factor in Preterm Infants With Respiratory Distress Syndrome. Pediatr Pulmonol. 2005 May;39(5):461-5. 15.Kenan S, Kimberly AD, Antonio Capone Jr, Michael T, Trese MT. Vitreous Levels of Stromal Cell–Derived Factor 1 and Vascular Endothelial Growth Factor in Patients with Retinopathy of Prematurity. Ophthalmology. 2008 Jun;115(6):1065-1070.e1. 16.Kandasamy Y, Hartley L, Rudd D. Vascular Endothelial Growth Factor-A Levels inTerm Neonates. J Child Sci. 2017; 7: e151-e154. 17.Gariano RF, Thomas WG. Retinal angiogenesis in development and disease. Nature. 2005 Dec 15;438(7070):960-6. 18.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. – 2015 Jan;122(1):200-10. 19.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012 Dec 27;367(26):2515-26. 20.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000 Apr;41(5):1217-28. 21.Lashkari K, Hirose T, Yazdany J, Wallace JM, Kazlauskas A, Rahimi N. Vascular Endothelial Growth Factor and Hepatocyte Growth Factor Levels Are Differentially Elevated in Patients with Advanced Retinopathy of Prematurity. Am J Pathol. 2000 Apr;156(4):1337-44. 22.Penna JS, Madanb A, Caldwellc RB, Bartolic M, Caldwellc RW, Hartnettd ME. Vascular Endothelial Growth Factor in Eye Disease. Prog Retin Eye Res. 2008 Jul;27(4):331-71. 23.Reddy B, Doddamani R, Koujalagi MB, Guruprasad G, Ashwini RC, Aradya GH, Raghoji C. Retinopathy of prematurity in a tertiary care hospital: incidence and risk factors. Int J Pediat Res. 2016; 3(5):364-70. doi:10.17511.ijpr.2016.5.16 24.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49(12):5177–82. 25.Smith LE, Wesolowski E, McLellan A. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. 26.Woo SJ, Park KH, Lee SY. The relationship between cord blood cytokine levels and perinatal factors and retinopathy of prematurity: a gestational age-matched case-control study. Invest Ophthalmol Vis Sci. 2013 May 15;54(5):3434-9. 27.Yenice O, Çerman E, Ashour A, Ridvan F, Goncagul H, Sirikci O, Akman I, Kazokoglu H. Serum Erythropoietin, Insulin-like Growth Factor 1, and Vascular Endothelial Growth Factor in Etiopathogenesis of Retinopathy of Prematurity. The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|